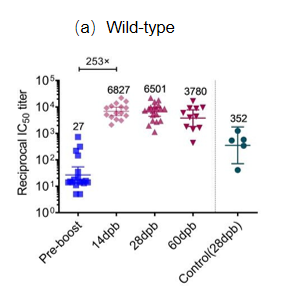
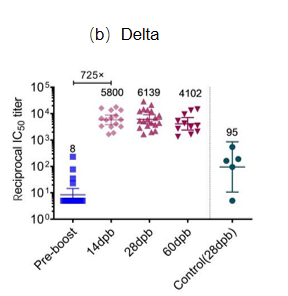
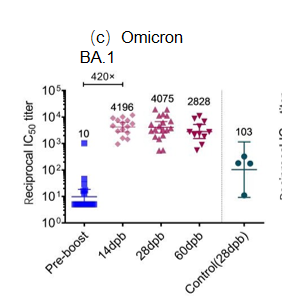
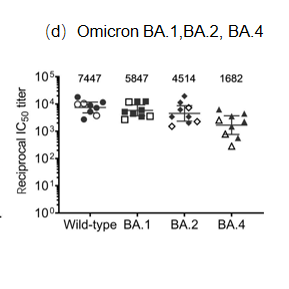
Background
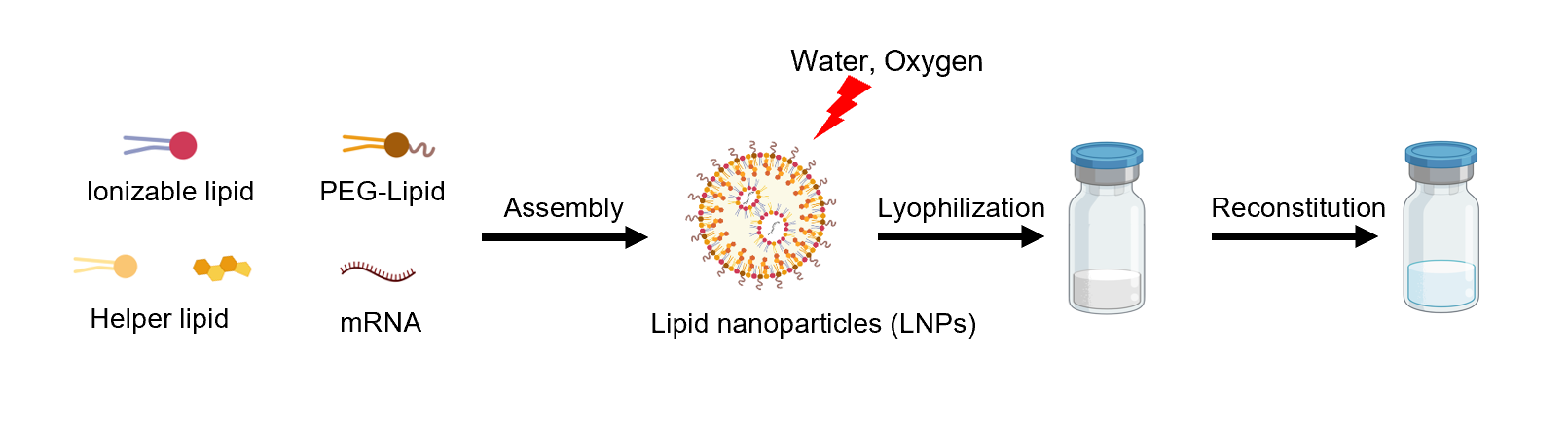
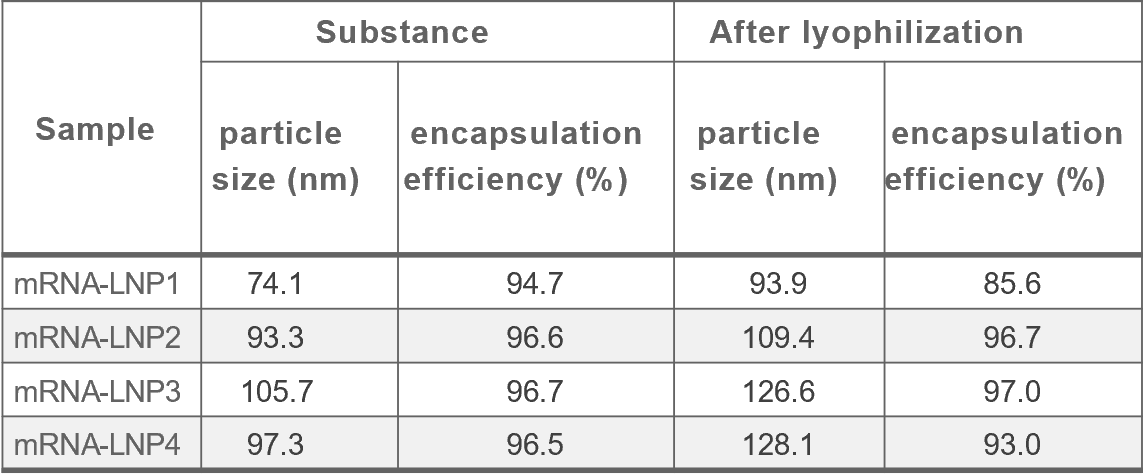
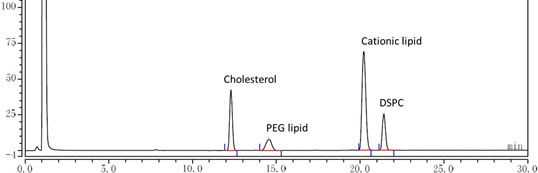
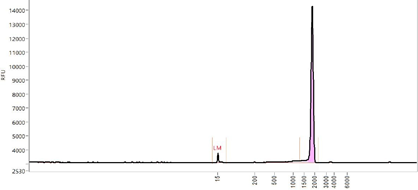
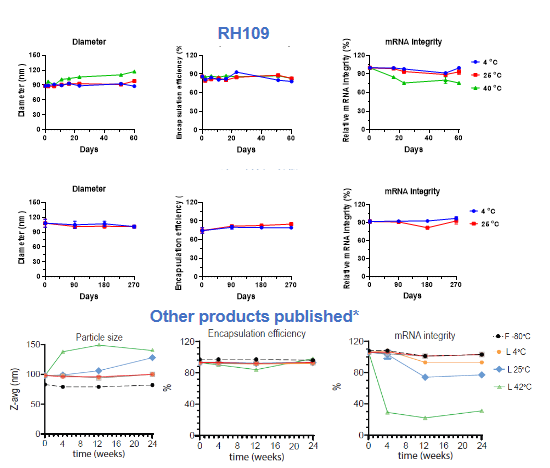
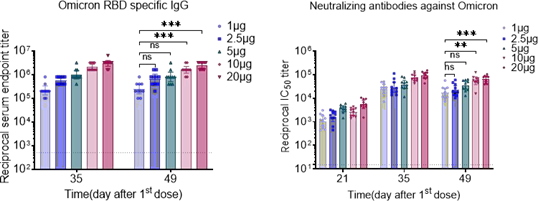
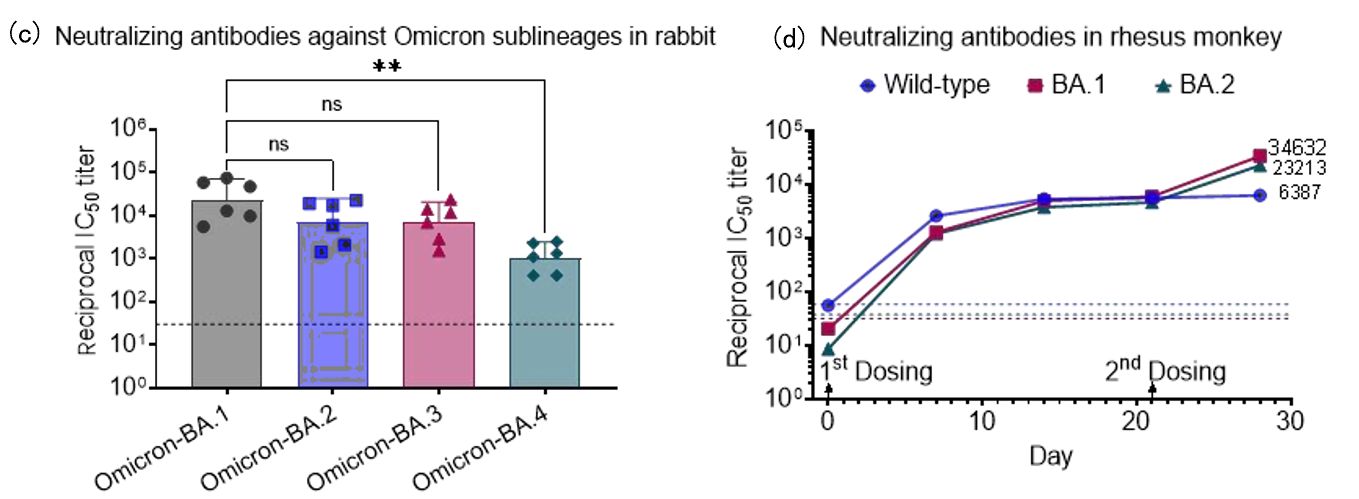
Cryogenic preservation and transportation are needed for the two current licenced mRNA vaccines (<-60°C). The stringent requirements come from the complex interactions among multiple lipid components and the instability of mRNA, which is sensitive to oxygen, moisture, enzymes, pH, and so on.
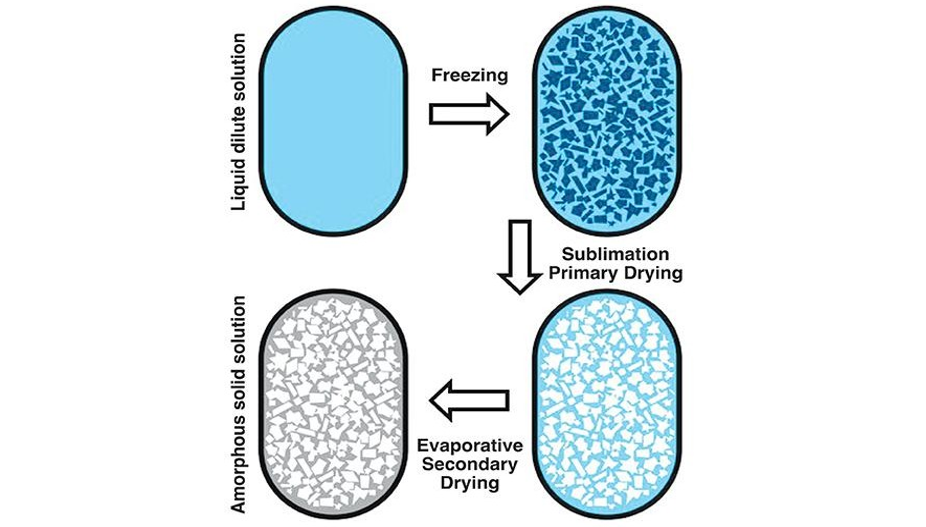
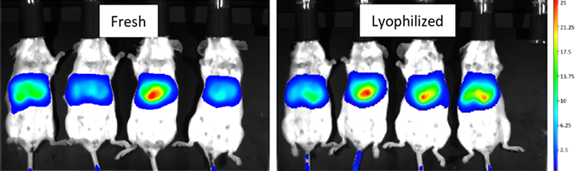
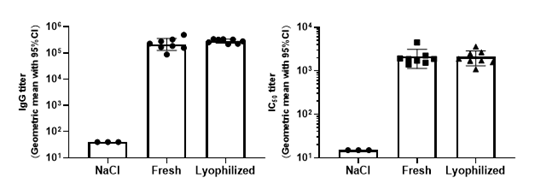
Lyophilization, a process that removes water by sublimation under vacuum at a low temperature, seems to be a promising solution. It is a relatively mild drying method applicable to vulnerable biomacromolecules or colloidal nanoparticles.