Process Development
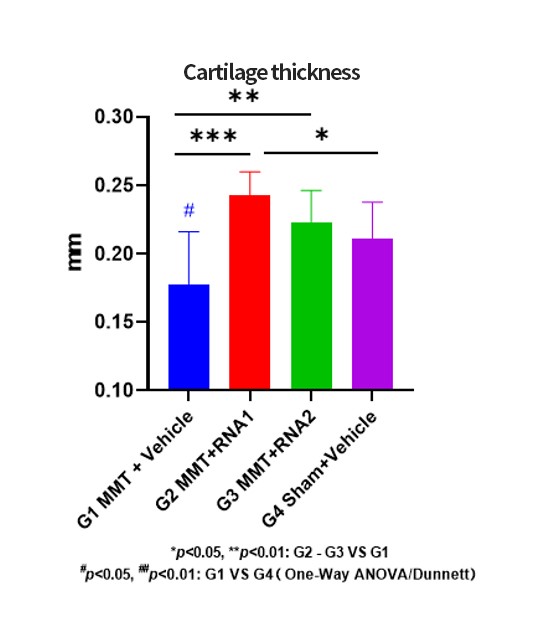
The process development work often takes place after preliminary research and before formal production. Early-stage research on the RSV project has shown promising feasibility of the candidate sequence in preclinical experiments. Therefore, it is necessary to develop related processes with the aim of efficiency, stability, and cost-effectiveness for future scale-up production. Through small-scale process development and validation, higher yields, purity, and cost-effectiveness can often be achieved.
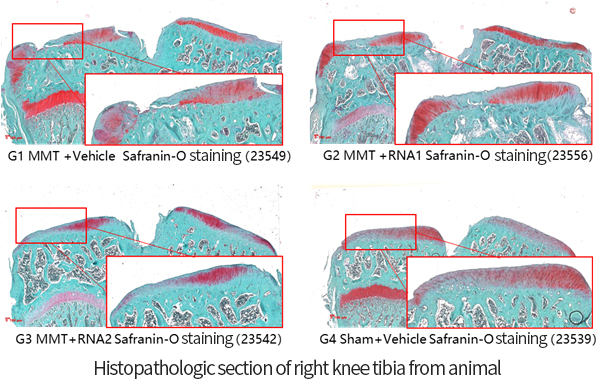
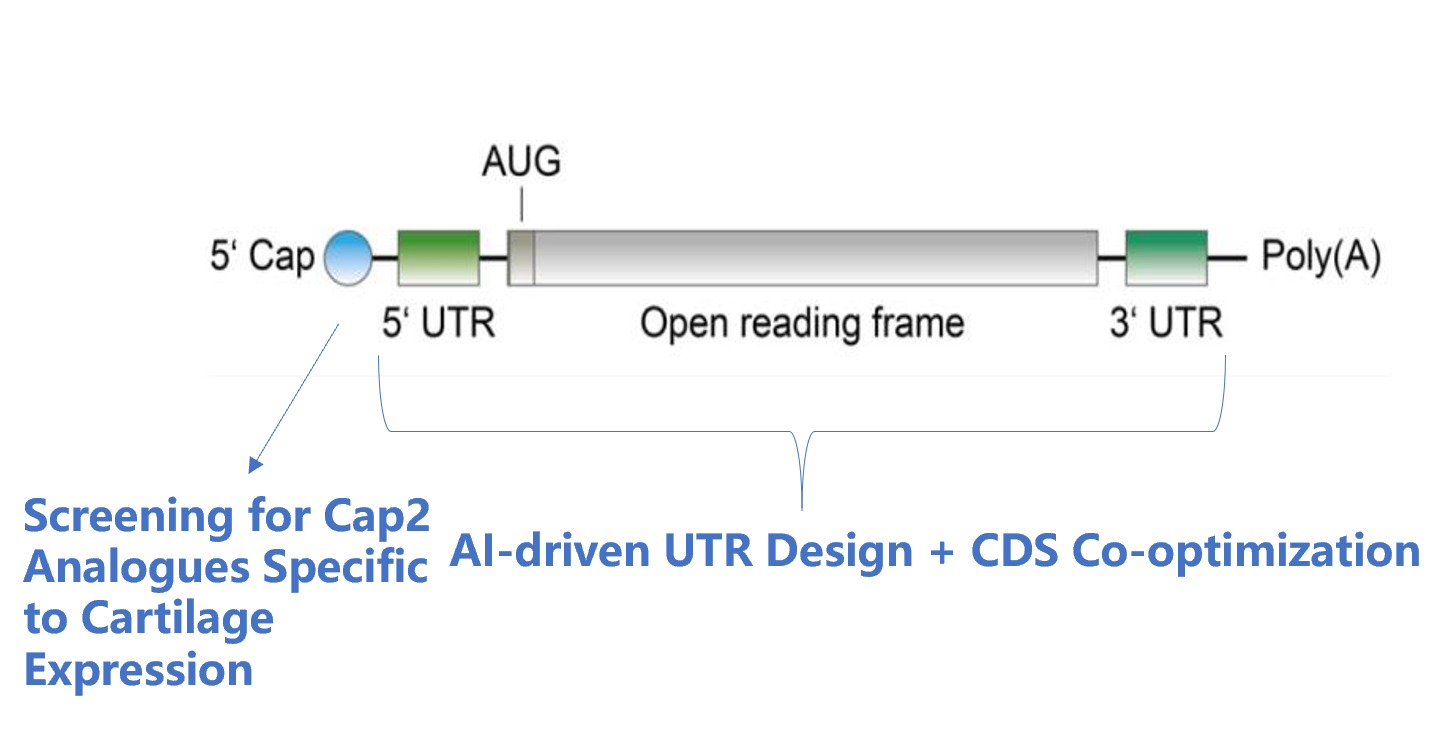
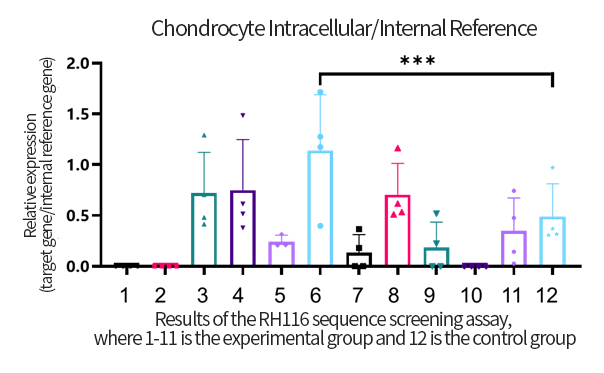
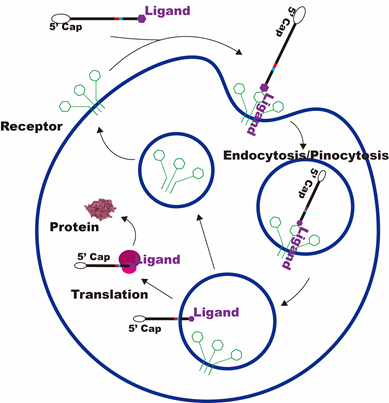
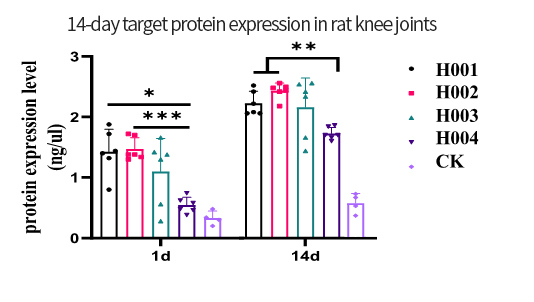
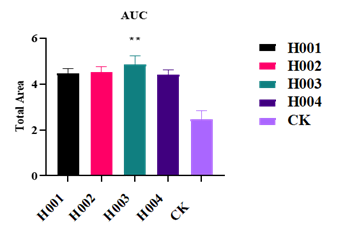
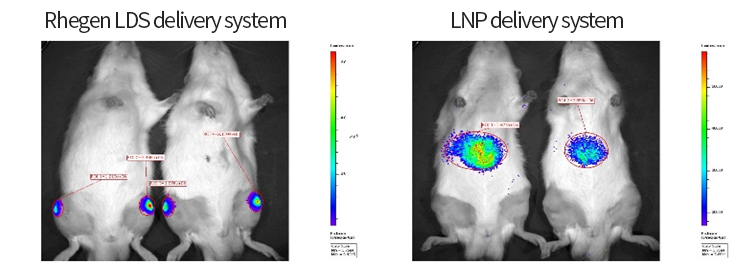
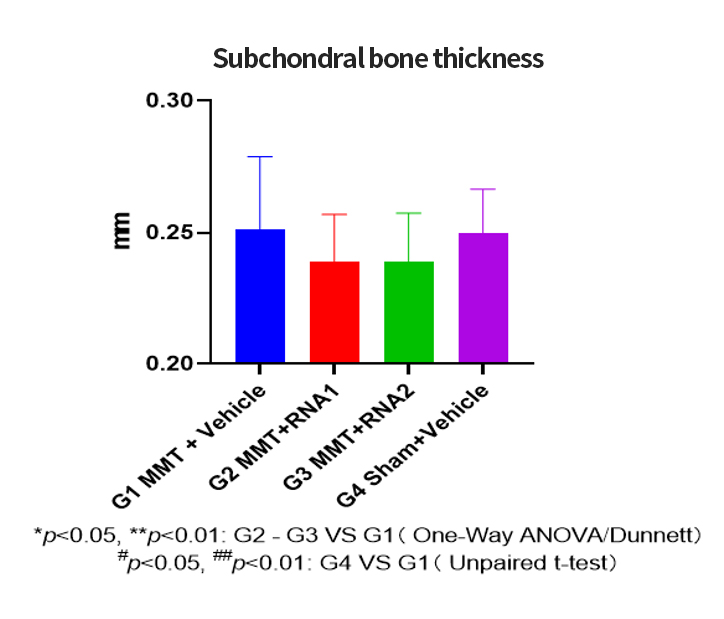
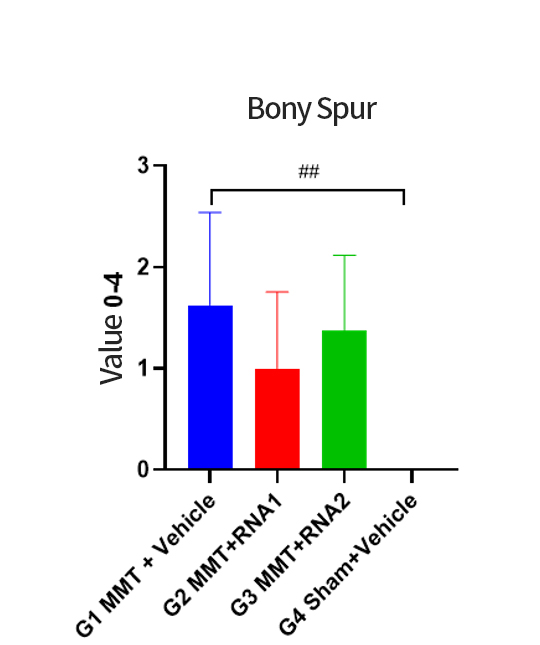
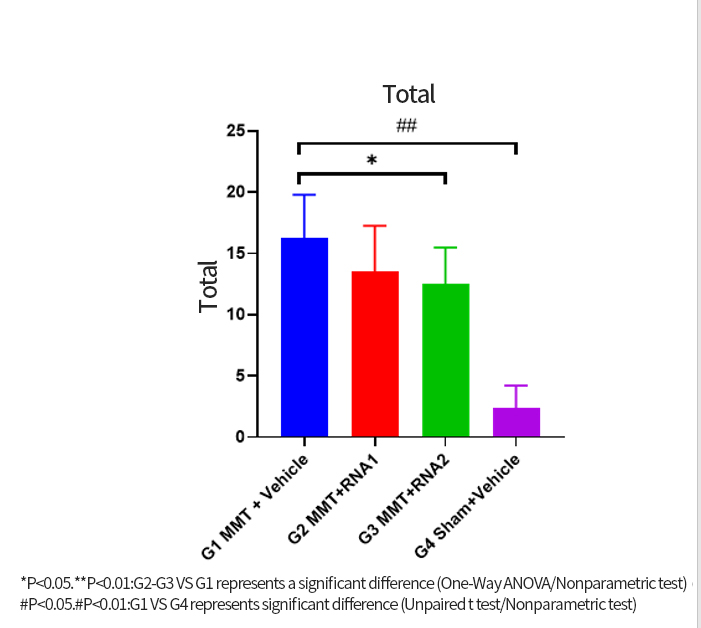
The OA project adopts the company's mature process platform, coordinating the compatibility between independently designed sequences and developed components. The process flow is optimized, streamlining and simplifying where necessary.
The plasmid template purity reaches >99%, with low endotoxin levels of <0.08 EU/μg.
For the stock solution, double-stranded residue is well below 0.04 μg/mg, with a capping efficiency exceeding 97%.