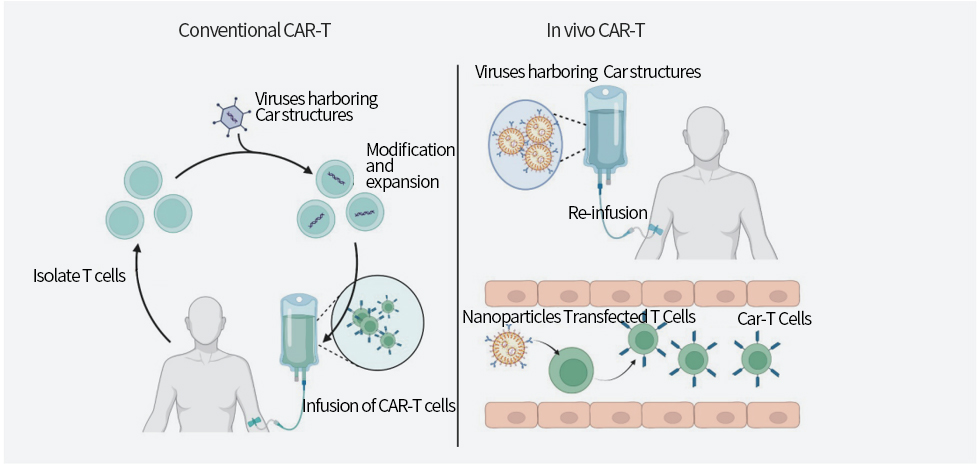
CAR-T therapy, as a promising new cancer immunotherapy method, has achieved significant success in the treatment of non-solid tumors and made breakthroughs in the treatment of solid tumors and other challenging diseases. However, the personalized preparation process of CAR-T and the high cost greatly limit its application. CAR-T mRNA drugs utilize the transient expression characteristic of mRNA to temporarily modify T cells in the body safely, transforming CAR-T technology from personalized therapy into a universal tumor drug. At the same time, it also reduces the risk of exogenous biological contamination, providing a new approach for cancer treatment.
EnoBio provides an integrated solution covering the entire value chain from CAR structure construction to clinical sample production and regulatory submission. Clients can choose integrated or single-module/technical point solutions based on their needs, including AI-guided CAR structure protein design (DMC.AI platform), CAR structure mRNA design and synthesis, safe and efficient transfection of T cells using targeted LNP delivery systems, immunostimulatory cap analogs, immunostimulatory UTR and poly A tails, etc. With the combination of proprietary technology, key raw materials, and R&D production equipment supply chain, EnoBio can not only solve the comprehensive cost issues of CAR-T mRNA drug products but also meet the development of various CAR structures and related drug needs with full control over the entire chain.