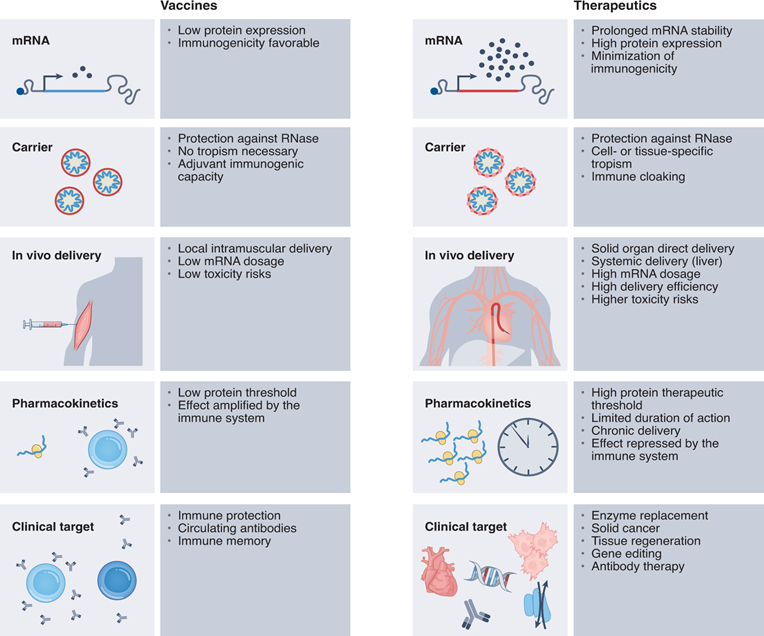
Immune boosting solution

Immune boosting program
1.Applicable to the research and development of vaccines for preventive and therapeutic infectious diseases or tumor vaccines.
2.mRNA can increase mRNA translation efficiency by sequence optimization and express target proteins of specific sequences to induce specific immune response .
3.Delivering mRNA to immune organs such as spleen or lymph nodes can enhance immune response.
4.As an immunogenic nucleic acid, mRNA can also induce the body's natural immune response, making it have the characteristics of "self-adjuvant".
5.Optimizing these three aspects can enhance the immunogenicity of the vaccine.
Modification of untranslated regions (UTRs)
There are untranslated regions (UTRs) at both ends of the coding region of mRNA, specifically known as the 5' UTR at the 5' end and the 3' UTR at the 3' end. These UTR sequences are not codons and cannot be translated into amino acids, but they can regulate the degradation and transcription efficiency of mRNA products through RNA-binding proteins. Therefore, the design of UTR components is crucial for every mRNA vaccine, aiming to enhance the stability and transcription efficiency of the mRNA vaccine in the target cells. Numerous studies have demonstrated that the UTR sequences of African clawed frog and human α-globin/β-globin are highly stable and can enhance the expression efficiency of heterologous mRNA. Consequently, the UTR elements of these two proteins are often utilized as UTR components in mRNA vaccines. However, UTRs from other proteins have also been studied for use in mRNA vaccine components (supplementary).
Exploration breakthrough: mRNA tissue-specific solution based on Cap2 analogues
Case: Application in vaccine development pipeline significantly enhances cellular immunity
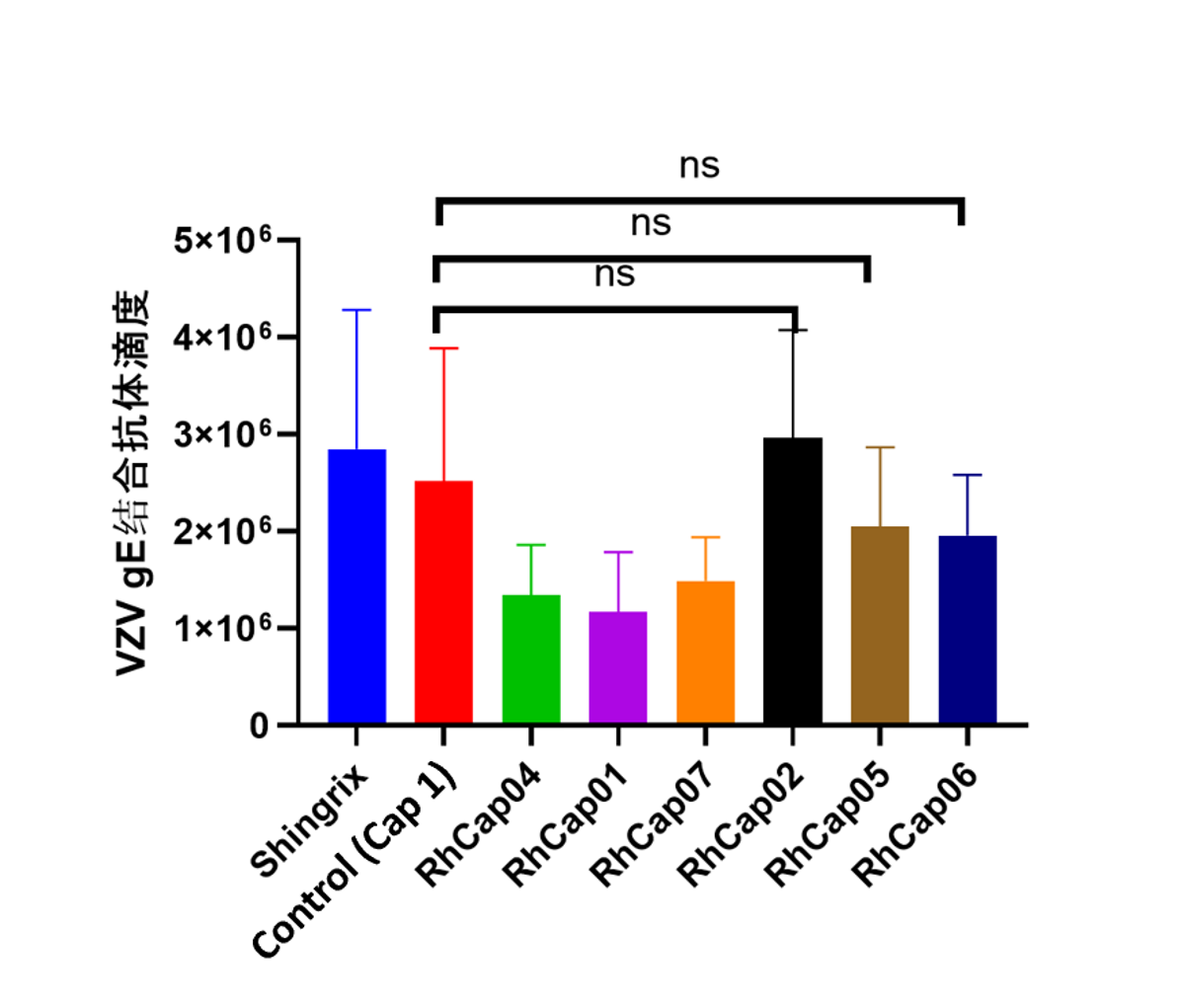
RhLNP-IE: High expression in the spleen, suitable for immune related pipelines
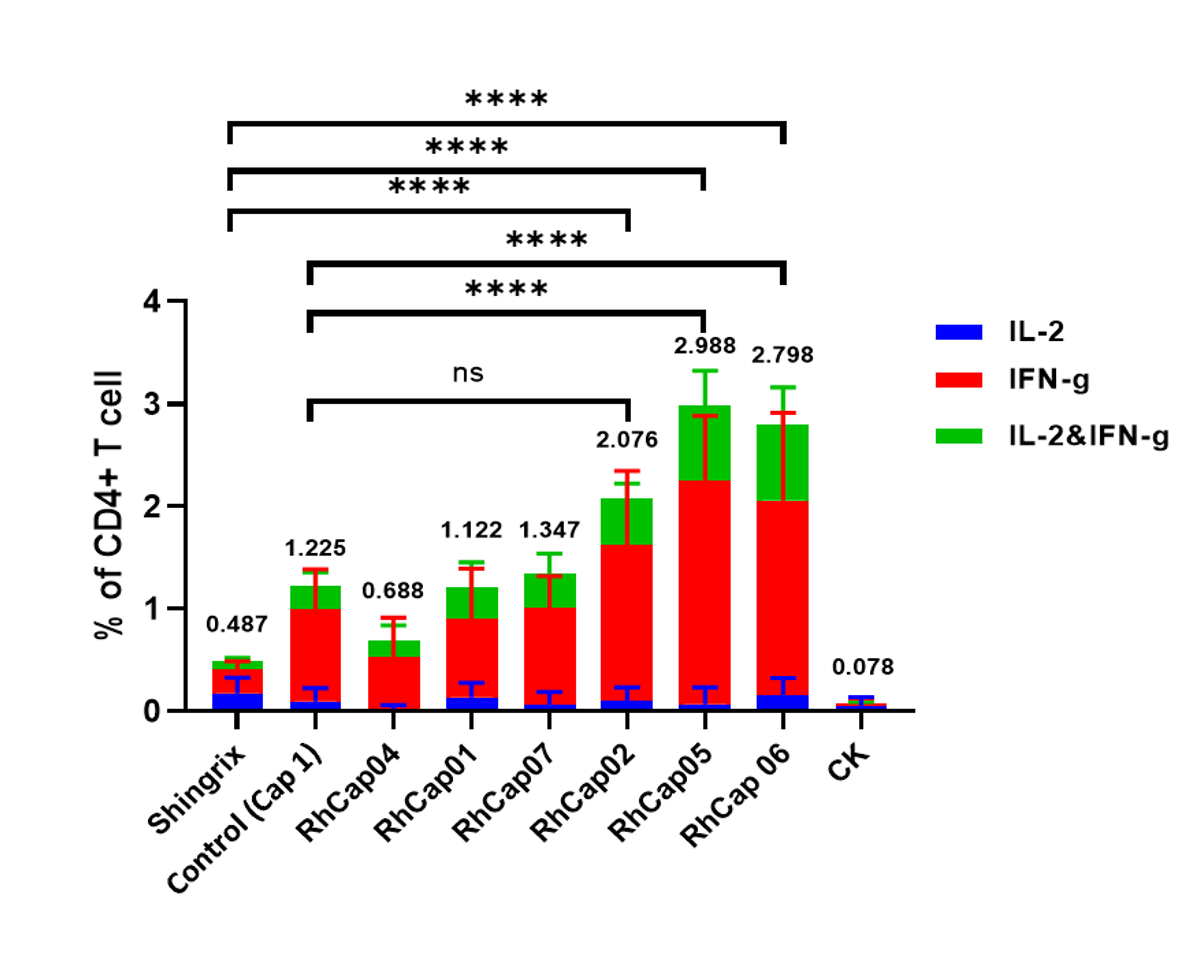
RhLNP-IE: High expression in the spleen, suitable for immune related pipelines
Exploring Breakthrough: Immune Enhancement LNP Solution
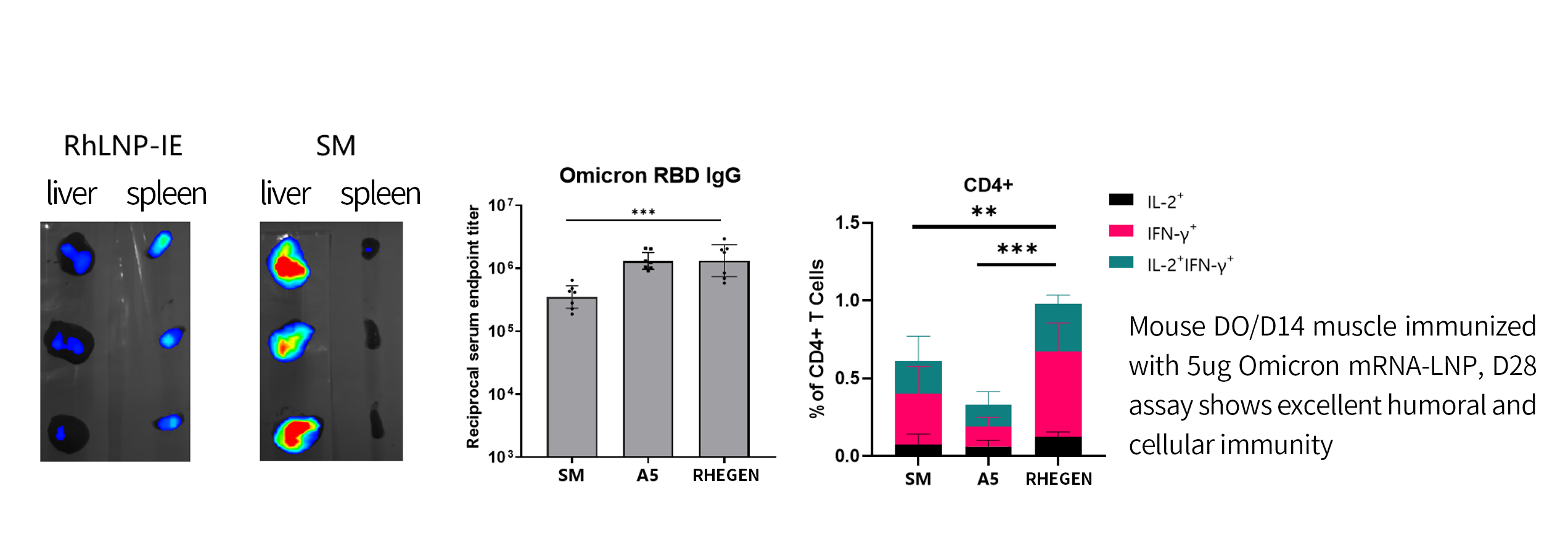
RhLNP-IE: High expression in the spleen, suitable for immune related pipelines
Exploration breakthrough: mRNA-LNP preparation solution
Case: Application in vaccine development pipeline significantly enhances cellular immunity

Liposyn X Suitable for dosage form screening (0.4-15 mL)

Liposyn Y Suitable for small-scale trial processes (1-100 ml)

Liposyn ZSuitable for industrial - continuous production(>100 mL)
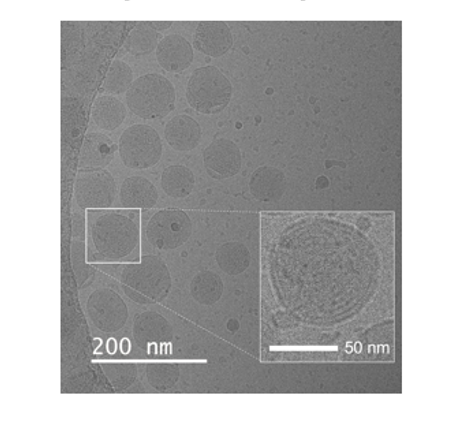
Electron microscopy image of LNP produced by Liposyn Z
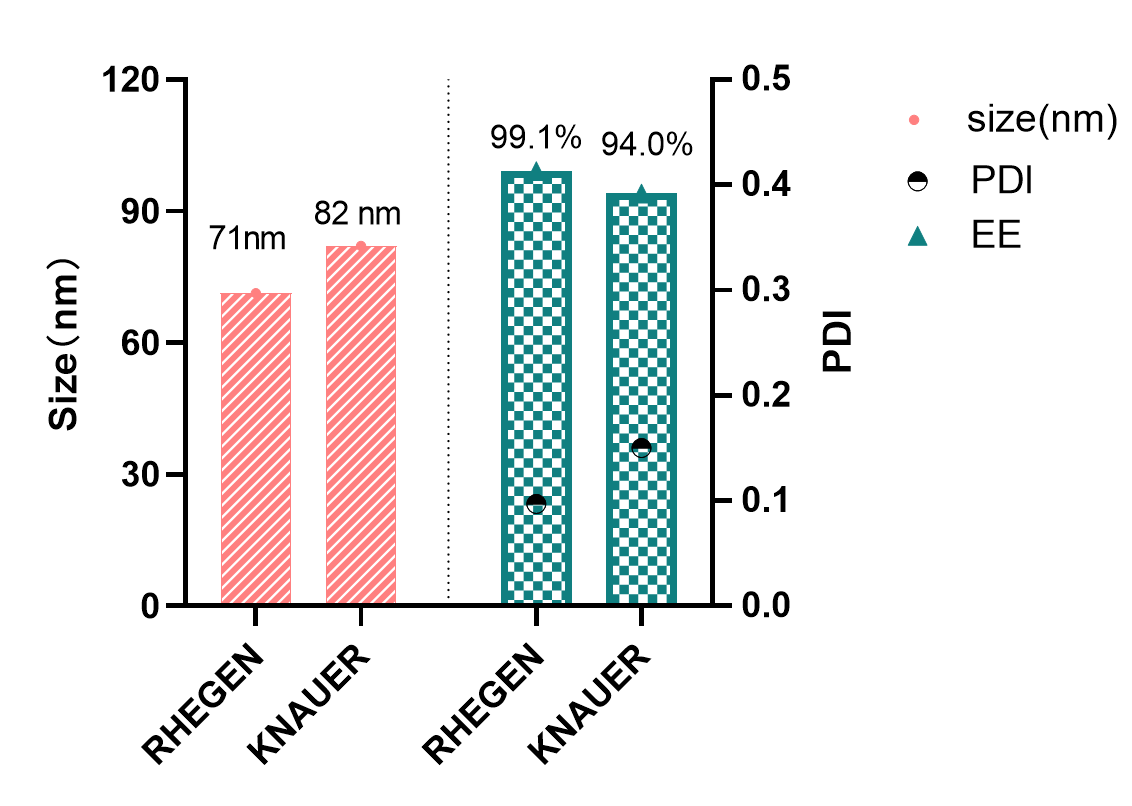
Excellent quality characterization parameters of LNP produced by LiposynZ