One-Stop mRNA Services
The one-stop service start with sequence synthesis. The optimized sequences will synthesis and cloned into the target plasmid. In this part, we provide multiple choices such as 5' UTR, 3' UTR and poly A to client for better expression level. The sequencing and gel electrophoresis are explored for quality test.
Featured business
Complex sequence synthesis
Vector construction
Support customers’ individual needs Can provide own carrier.
Order quantity
Regular: 0.1-10 mg Support other specifications
Component selection
UTR Poly A Cap Pseudouridine.
Purification method
LiCl precipitation, kit purification.
QC
Standard testing Support customers’ individual needs
In Vitro Transcription mRNA
Messenger RNA (mRNA)-based drugs innovation have been widely proven as a promising treatment strategy in immune therapeutics. Our mRNA one-stop service obtained synthesis, sequencing, IVT and evaluation. The fast, efficient and comprehensive mRNA services will help your drug innovation, overcome experiment challenges and time saving in the competitive market environment.
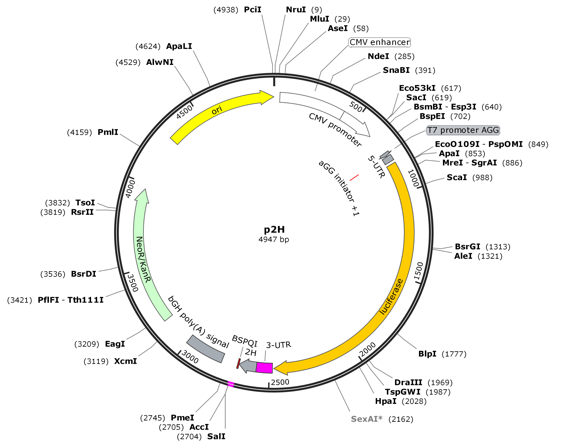
Gene synthesis and vector construction
Gene synthesis service: it has a complete experimental operation process and high-difficulty sequence synthesis solutions, and the success rate and delivery rate of gene synthesis service can reach 99.32% and 95%. It has a leading advantage in the difficulty of DNA synthesis and quality testing for sequences rich in repeated sequences, high GC content, hairpin structures, and continuous single bases.
Synthesis and build cycle
| sequence length | synthesis cycle | remark |
|---|---|---|
| 0~400bp | 7 | regular sequence |
| 400bp~1kb | 7-10 | regular sequence |
| 1kb~2kb | 8-13 | regular sequence |
| 2kb~3kb | 12-17 | regular sequence |
| 3kb~4kb | 16-20 | regular sequence |
| 4kb~5kb | 20-25 | regular sequence |
| 5kb~6kb | 25-30 | Consultation larger than 6kb, delivered in fragments |
| service type | length | build cycle |
|---|---|---|
| 5'UTR vector construction | <0.5kb | 5-10 working days |
| 3'UTR vector construction | <0.5kb | 5-10 working days |
| Construction of Poly(A)tail vector | <0.2kb | 5-10 working days |
| CDS vector construction | <2kb | 5-10 working days |
| >2kb | For every additional 1kb, add 3 working days | |
| Other types of plasmids | / | Negotiate with customers |
Quality Inspection
The synthesized and constructed vectors are judged by agarose gel electrophoresis to determine whether the plasmid size is as expected. After the first-generation sequencing, sequence alignment is performed to confirm the target vector.
In vitro transcription
In order to meet the different needs of customers, Enobio Biotechnology provides mRNA synthesis services ranging from micrograms to milligrams. Under the catalysis of T7 RNA polymerase, using four kinds of nucleotides as raw materials and linearized DNA as templates, we imitate the process of transcription in vivo to obtain single-stranded mRNA by transcribing double-stranded DNA.
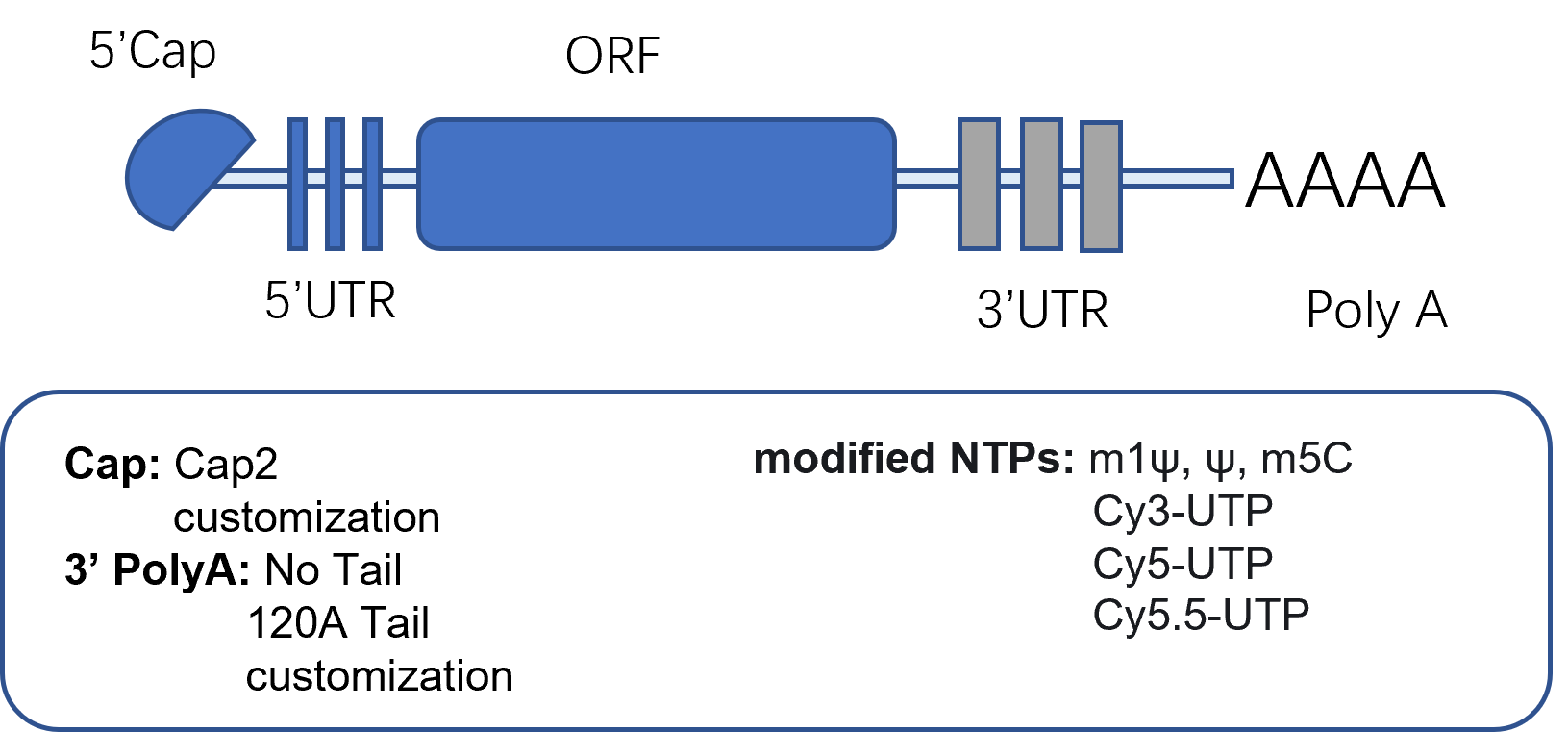
Cycle and quality control
| laboratory level | |
|---|---|
| application | basic research applications, new drug research and development, preclinical research, etc. |
| specification | mRNA: 0.1-10mg |
| cycle | IVT mRNA production: 7~14 days |
| quality specification system | ISO9001 |
| production facilities | parallel production in shared laboratory areas |
| document control and traceability | no |
| QC project and release | QC |
| reserve sample | available on demand |
| additional deliverables | COA agreement |
| QC | Item | Normal | Upgrade |
|---|---|---|---|
| physicochemical property | Appearance | √ | √ |
| PH | x | √ | |
| Identification | Length of mRNA(CE) | x | √ |
| Length of mRNA(electrophoresis) | √ | x | |
| sequencing | x | √ | |
| Content | Content of mRNA | √ | √ |
| Purity | Purity(CE) | x | √ |
| Purity(electrophoresis) | √ | x | |
| Purity(HPLC) | x | √ | |
| A260/A280 | √ | √ | |
| capping efficiency | x | √ | |
| Length of poly A | x | √ | |
| Impurity | protein | x | x |
| Template | x | x | |
| dsRNA | x | x | |
| Security | endotoxin | x | x |
| bioburden | x | x |

Evaluation-In vitro activity verification of mRNA
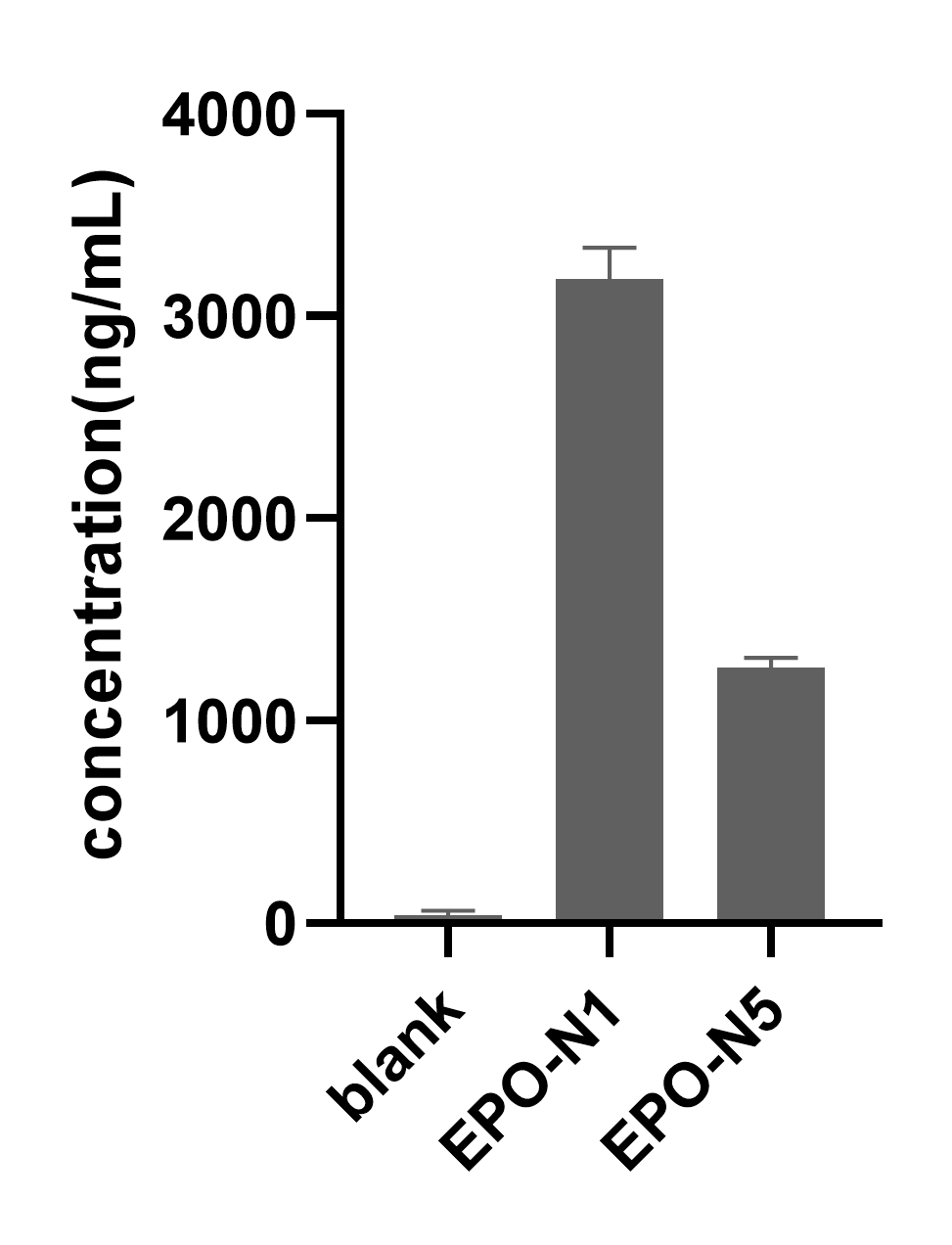
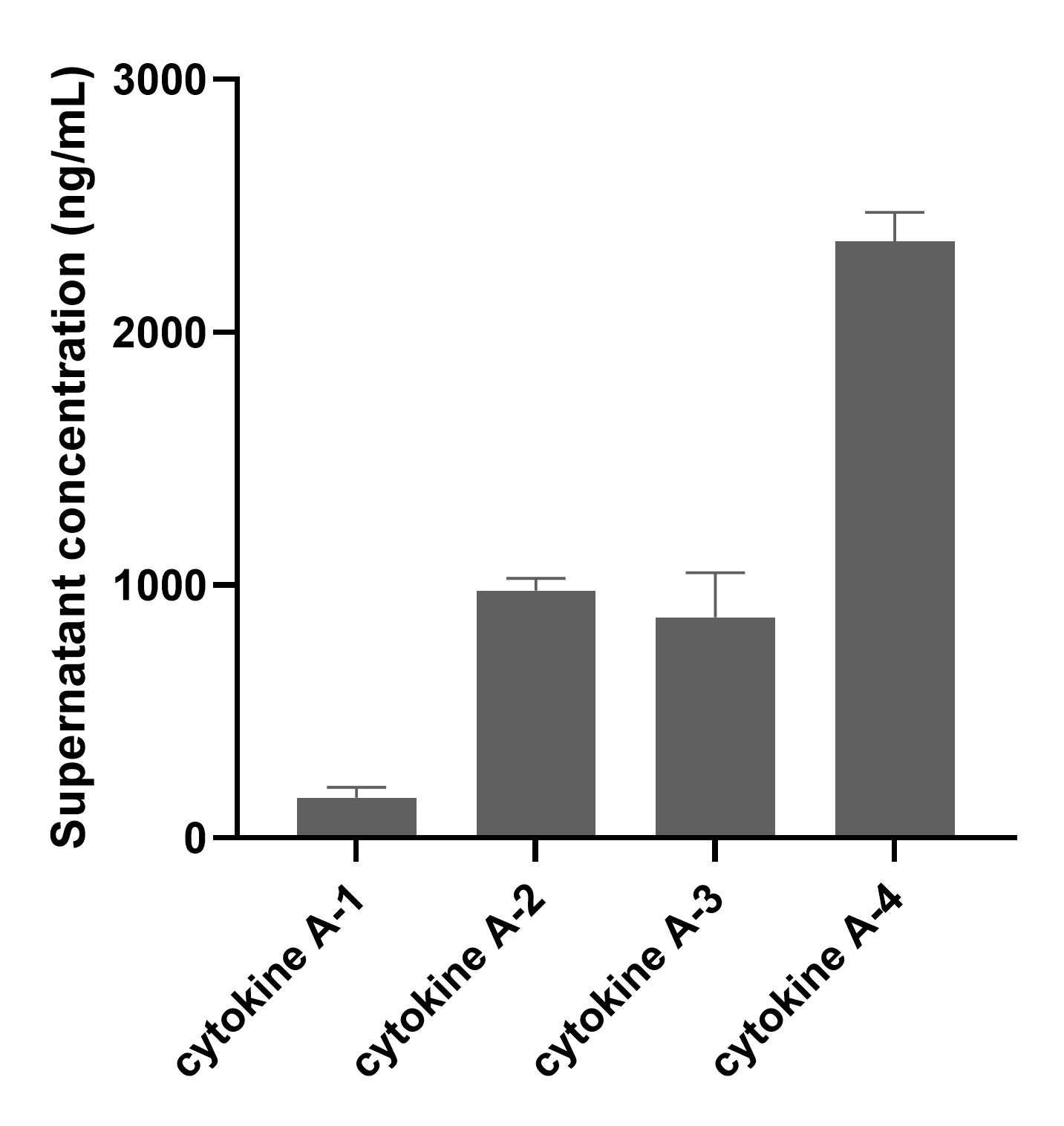
Different CAP structures, UTR sequences, nucleotide modifications, and CDS sequences can all affect the expression level of mRNA. The transfection and expression efficiency of mRNA can be evaluated by detecting the expression or activity of the target protein using methods such as flow cytometry, western blot, and ELISA.
Flow cytometry (FCM) detection
2E5 293T/17 cells were plated in a 24-well plate and cultured for 18-24 hours. Then, 1 μg of differently nucleoside-modified eGFP-mRNA was transfected using jetMESSENGER transfection reagent. After 18-24 hours, fluorescent microscopy and flow cytometry were performed to detect the transfected cells.
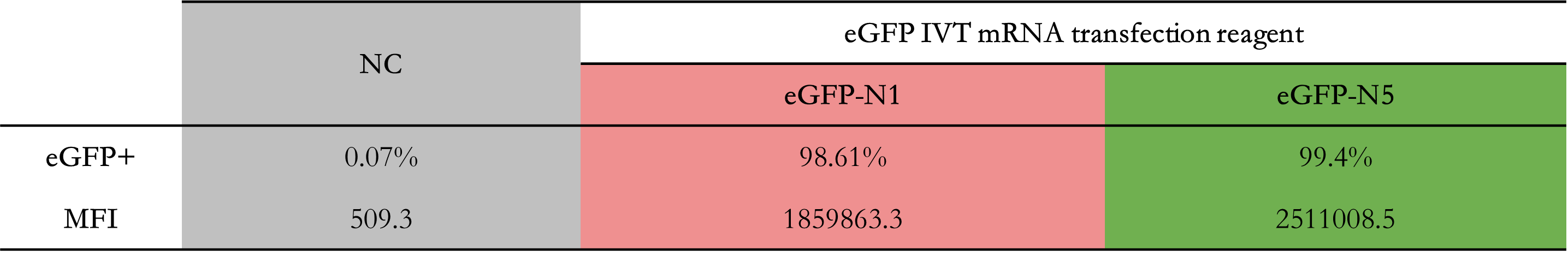
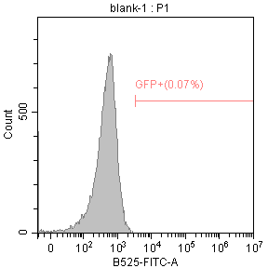
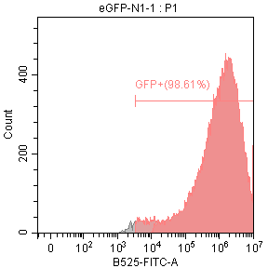
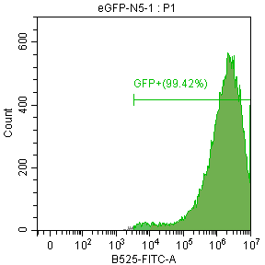

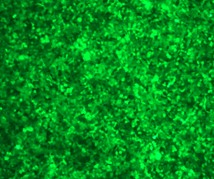
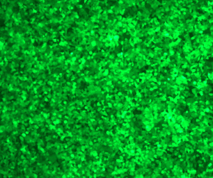
Enzyme-linked immunosorbent assay (ELISA)
CMC and Industrialization
CMC is an important part of new drug registration, mainly involving four modules in mRNA therapeutics areas: seed lot establishment, plasmid preparation, mRNA synthesis, and LNP preparation and filling. Among them, product quality, impurity control, process control, and process stability are all key points for mRNA therapeutics development.

Plasmid process development
We have a stable and reliable fermentation and plasmid purification process with comprehensive process control, clear and traceable records. The DNA template product shows high purity (>90%), low recombination rate (<5%), low endotoxin (<0.1EU/μg). We can provide a full set of DNA template analysis services.
mRNA Process Development
Using high-fidelity T7 polymerase, we offer a stable and reliable mRNA synthesis and purification process, with comprehensive process control, clear and traceable records. Our mRNA products show high purity (>90%), high capping efficiency (>90%), and low double-stranded residue levels (<1μg /mg). We can provide a complete set of mRNA analysis services.
In vitro transcription reaction
Template digestion
Purification
Sterilizing filtration
Formulation process development
We offer stable and reliable mRNA-LNP preparation and purification process development services, with comprehensive process control, clear and traceable records. The frozen formulation can be stored at <-60℃ for over 2 years, with an encapsulation efficiency of>85% and a particle size of <150 nm. Enobio's unique LNP freeze-drying process can meet the long-term storage needs of various combinations of mRNA and LNP, with a shelf life of>1 year at 2-8℃ for storage and transportation. The product shows high encapsulation efficiency of >80% with a particle size of <150 nm and can be easily reconstituted prior to use, providing high convenience and accessibility. We can provide a complete set of mRNA-LNP analysis services.
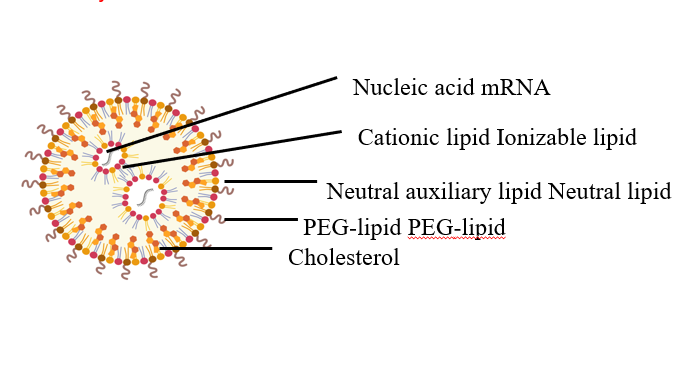
LNP preparation
Ultrafiltration purification
Semi-finished product preparation
Sterilizing filtration
Freeze/lyophilization
Analytical service
Enobio provides comprehensive analytical services for mRNA and its related products. Our analytical services encompass method development and validation, QC testing and release, as well as stability studies. We have extensive instrumentation for a broad range of analytical methods, and our team consists of recognized experts in mRNA analysis. With a robust quality assurance system meeting cGMP regulations, we ensure data integrity. Our analytical capabilities include identity, purity, impurity profiling, characterization, and safety testing for plasmid, mRNA, and mRNA-LNP programs.
Analytical method Development
Enobio will first assess the customer's product or service to determine if a new testing method needs to be developed. If a new method is needed, our method development team will be responsible for its development. After the method development is completed, it will be validated by the method transfer team. Once validated, it will be transferred to the QC team for quality control testing.
Quality control of Related products & services and anylatical method
Quality control of Plasmid
Plasmids are the starting materials for mRNA synthesis, and their quality directly impacts the quality of the mRNA. It is essential to assess sequence accuracy, supercoiling, and residual DNA/RNA from host bacteria.

Quality control of mRNA
The sequence accuracy, purity must be confirmed for the mRNA, along with critical structures such as the Cap and poly A tail. It is also essential to control impurities such as dsRNA and residual DNA templates.

Quality control of mRNA-LNP
The encapsulation efficiency of mRNA within lipid nanoparticles (LNPs) directly impacts the effectiveness of the mRNA product. It is crucial to perform quality control on key attributes, including encapsulation efficiency, particle size, and PDI (Polydispersity Index).

Stability studies
We can rigorously assess the quality changes of mRNA-related products under different conditions, such as temperature, humidity, and light exposure, strictly following the ICH guidelines.