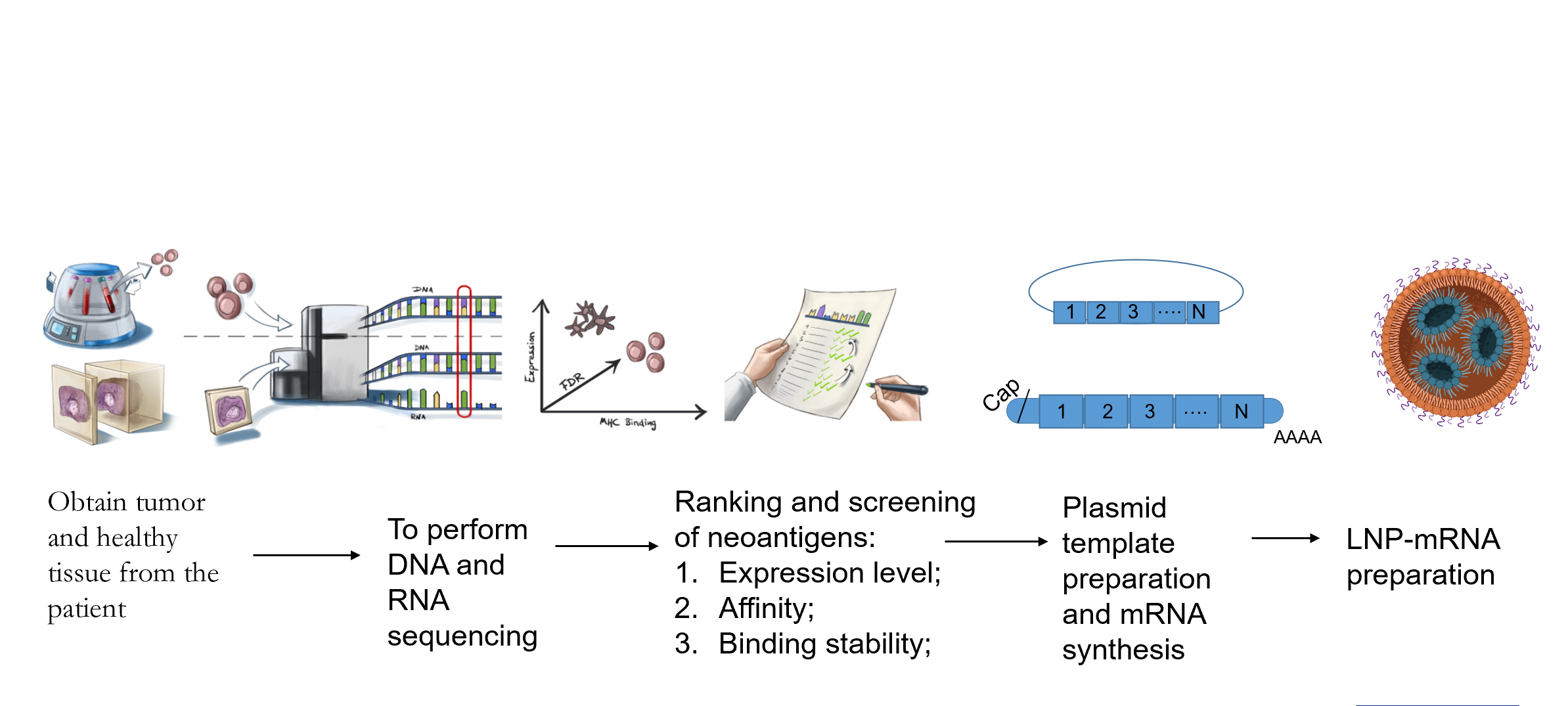
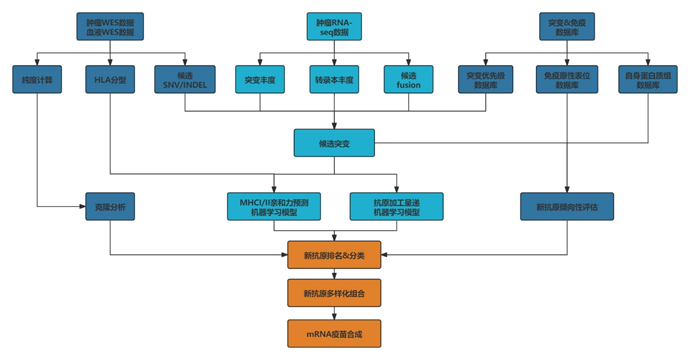
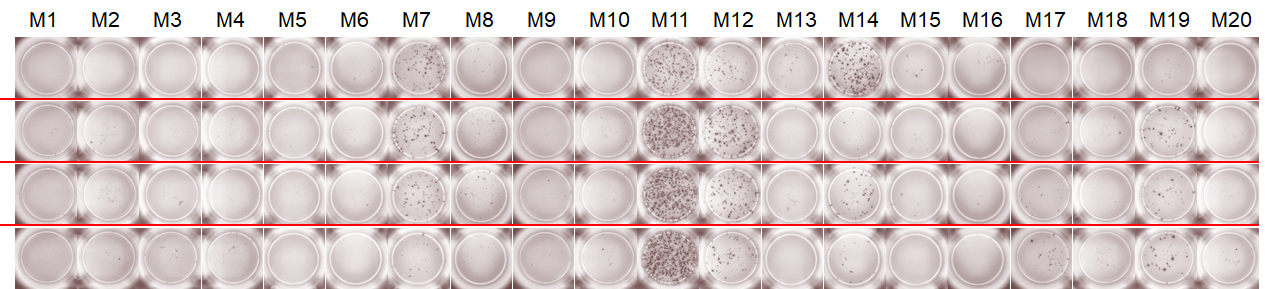
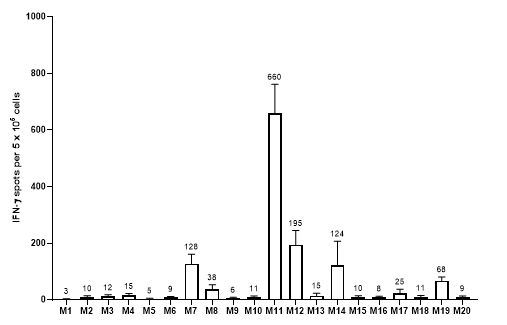
Personalized Cancer Vaccine CMC Challenge
1.Personalized preparation : Since the tumor mutation antigens of each patient are different, personalized tumor vaccines need to be customized according to the tumor characteristics of the patient, which requires a highly flexible production process to adapt to the needs of different patients .
2.Production efficiency and cost : In order to meet the treatment window, the production cycle from receiving the patient's sample tissue to completing the personalized tumor vaccine needs to be within 6-8 weeks or even shorter. Improving production efficiency and reducing costs is an important challenge to make this treatment method more widely accessible .
3.quality control : Production of personalized tumor vaccines requires strict quality control to ensure the safety, purity, and effectiveness of each vaccine batch. It is necessary to establish a highly standardized production process and formulate effective quality monitoring strategies.
4.stability : Personalized tumor vaccines need to maintain stability throughout the entire production and delivery process to ensure that they still have therapeutic effects when used. This requires in-depth research on the stability of the vaccine and the adoption of corresponding measures to protect the vaccine from environmental impacts.
5.Regulatory requirements : The production of personalized tumor vaccines must comply with strict regulatory requirements, ensuring that the production process meets relevant regulatory standards and obtains necessary approvals and licenses.
Eno Bio can provide independent intellectual property solutions covering the entire value chain from tumor antigen screening and prediction to clinical sample production and registration declaration. Customers can choose the following integrated or single-module/technology solutions according to their own needs, including: tumor neoantigen prediction, combined with multi-cistronic mRNA design and synthesis (multiple antigen expression), immune-enhancing capping analogs, targeted LNP delivery systems, immune-enhancing UTRs, and poly A tails.