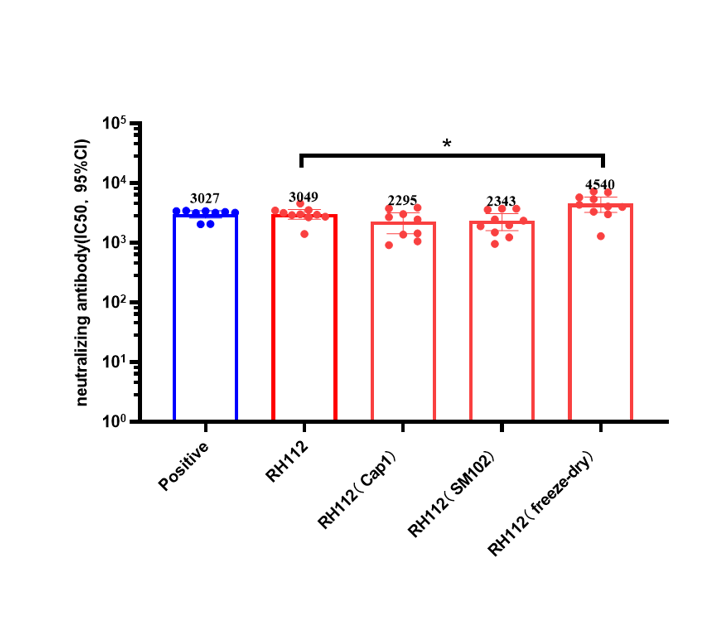
Respiratory syncytial virus vaccine: vast blue ocean market
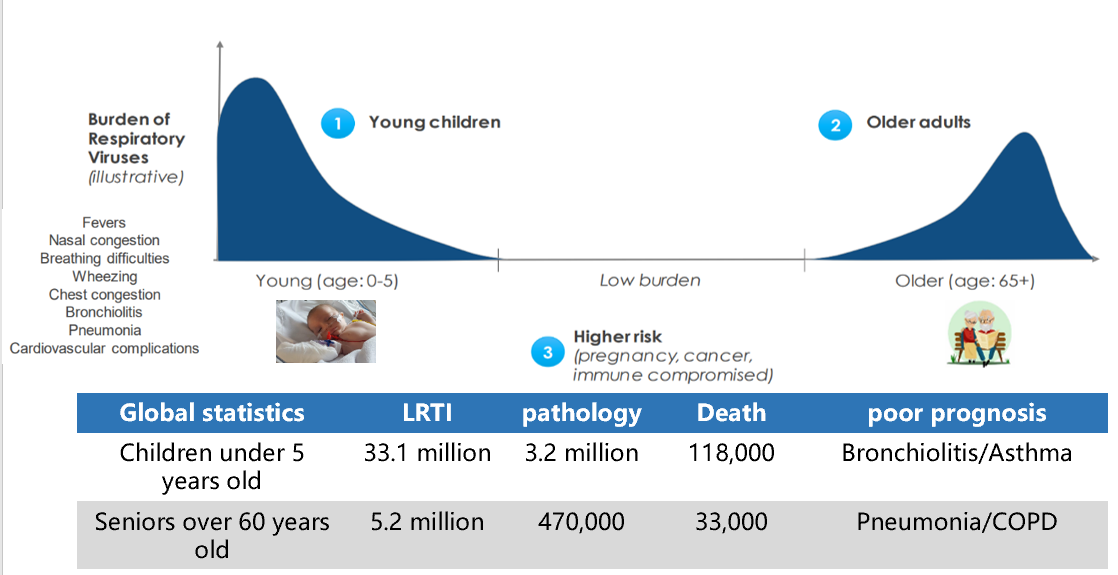
The burden of the disease is concentrated in immunocompromised individuals, lacking specific prevention and treatment methods, which has caused great clinical burden and social harm
RSV infection is epidemic among children under 5 years old, the elderly over 65 years old, and immunocompromised individuals. It causes tens of millions of infections and tens of thousands of deaths every year, resulting in a serious burden of disease.
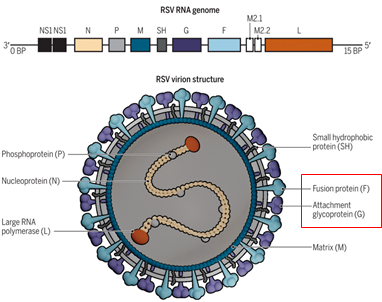
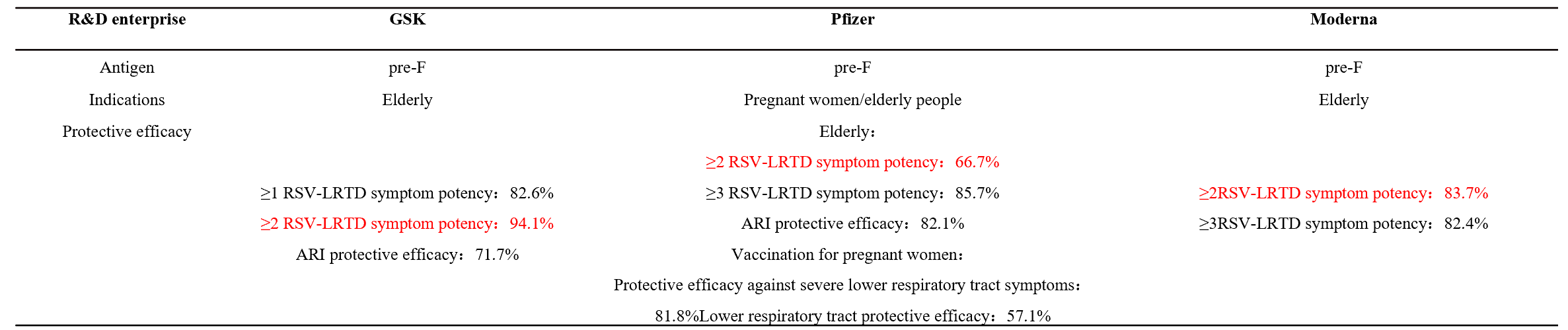
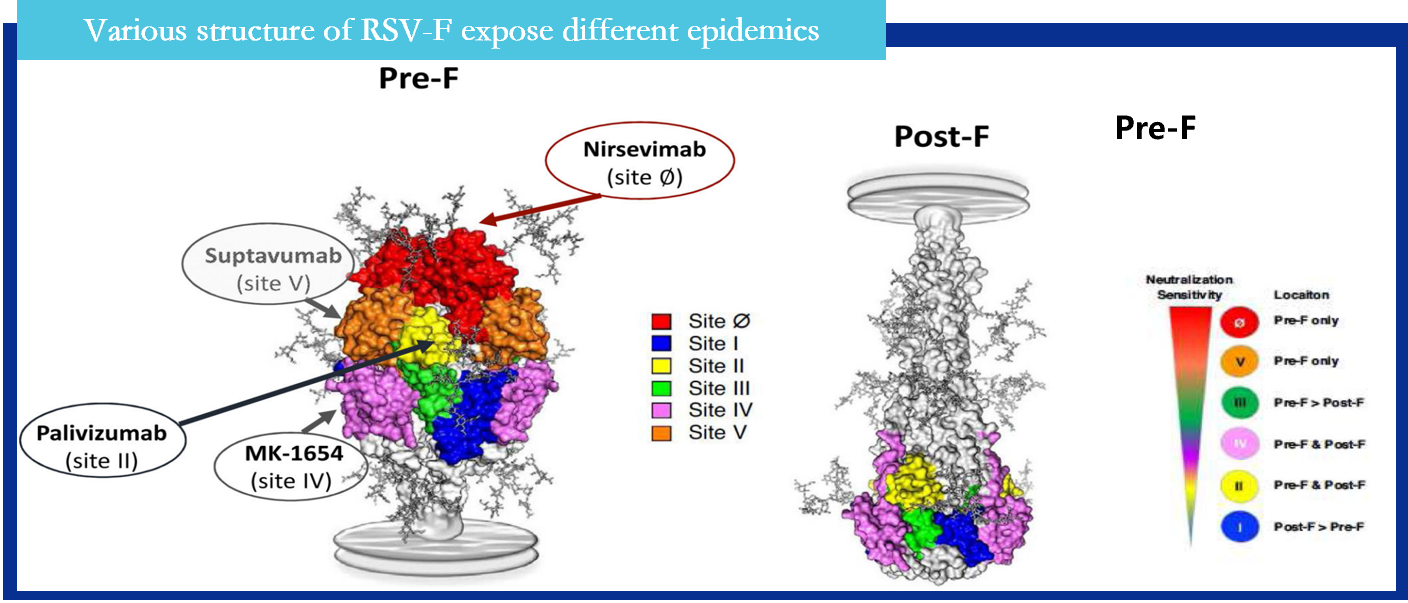
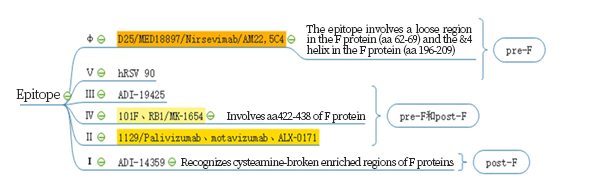
Respiratory syncytial virus (RSV) is a worldwide epidemic virus and cause serious social burden. In decades, research results shown that the prefusion structure of the RSV F glycoprotein has more epitopes compared with postfusion RSV F.