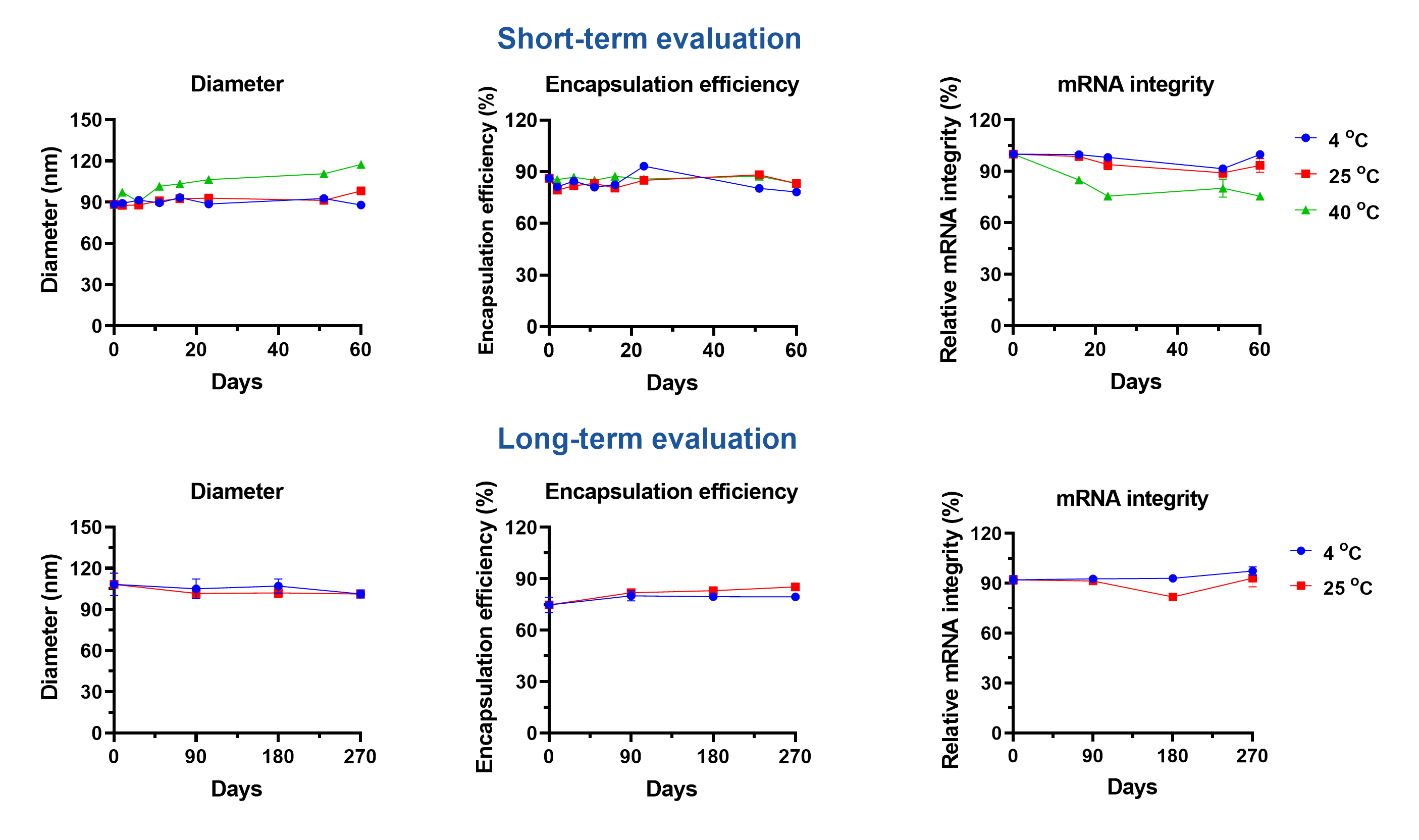
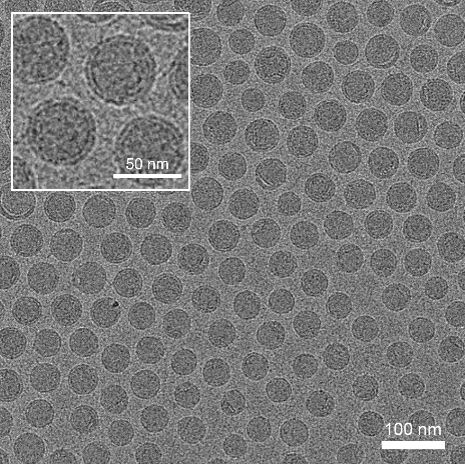
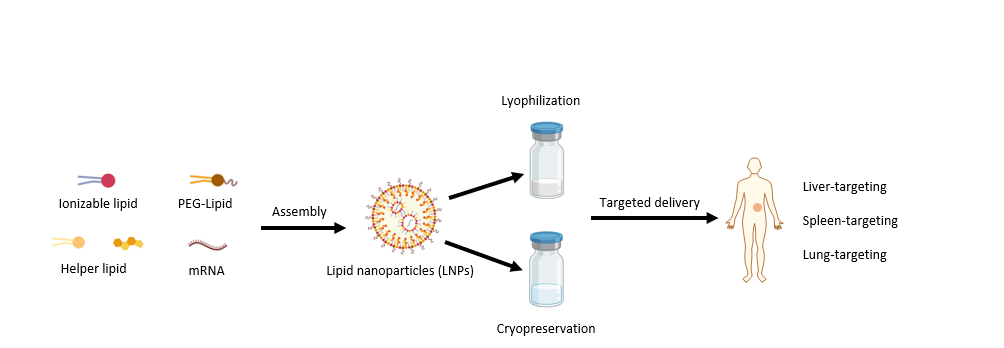
Several lipids form lipid nanoparticles (LNP) carrying mRNA to targeted organelles or tissues, improving their in vivo transfection efficacy. Although mRNA therapeutics show high potency in many areas, challenges in specific-targeted delivery and poor physiochemical stability still impede their accessibility. Cryogenic preservation and transportation are needed for the two current licensed mRNA vaccines, BNT162b2 (-80~-60°C) and mRNA-1273 (-20°C).
Enobio provides novel LNP design (for liver-, spleen-, or lung-targeted delivery), encapsulation (customized service), and lyophilization services (with high storage stability). Enobio also provides proprietary catalog products such as patented cationic lipids, LNP encapsulation kits for different organ targeting, fluorescent lipid, and LNP transfection kits loaded with indicator mRNA (such as luciferase and green fluorescent protein mRNA).
Equipment for mRNA-LNP preparations
Providing mRNA-LNP preparation equipment with full specification coverage to meet all requirements (from research to CMC production)
LNP for targeted delivery
ENOLNP-L LNP: High expression in the liver, suitable for protein replacement therapy or liver disease-related pipelines.
ENOLNP-S LNP: High expression in the spleen, suitable for immunology related pipelines.
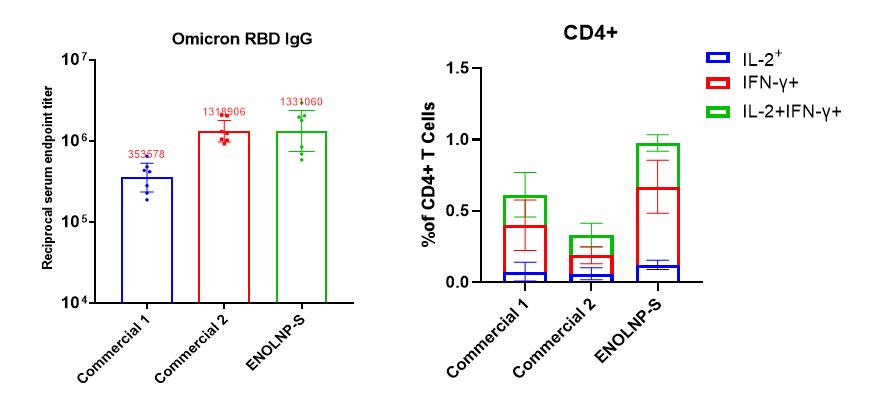
Intramuscular injection of 5μg Omicron mRNA-LNP (D0/D14) into mice, at D28 it showed excellent humoral and cellular immune responses.
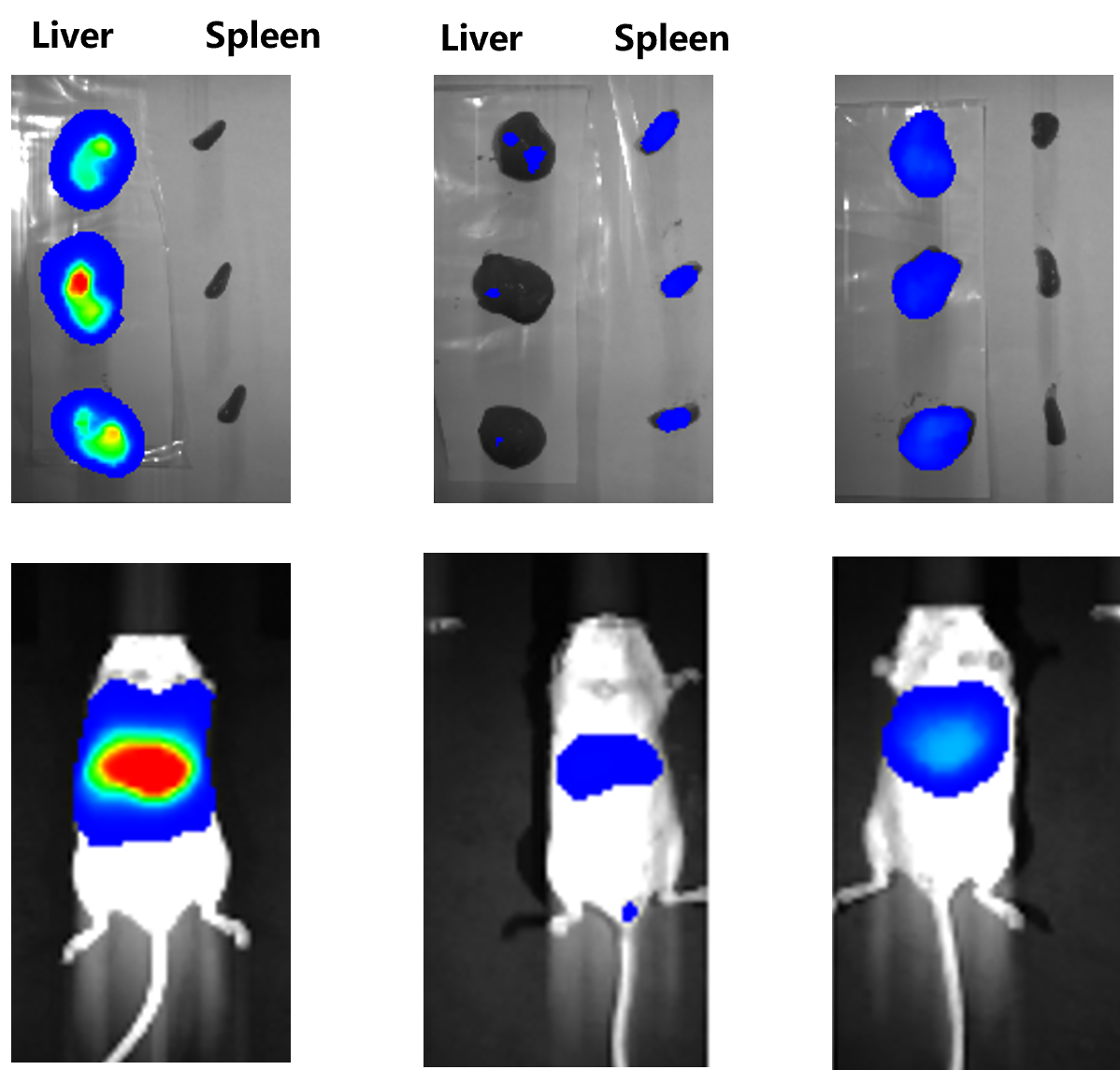
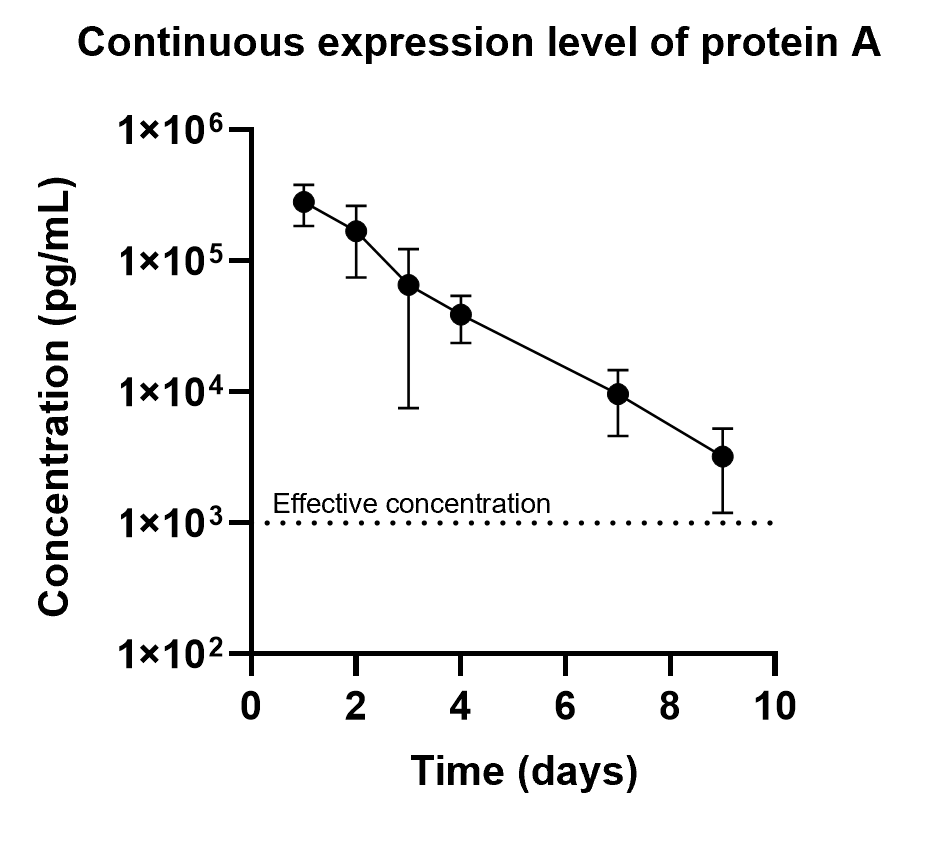
Injections intravenously 10μg of protein A's mRNA-LNP into mice, blood was collected and at different time points. It showed this formulation could achieve sustained expression of protein A for up to 9 days, while the half-life of the protein A itself is only 2 hours.
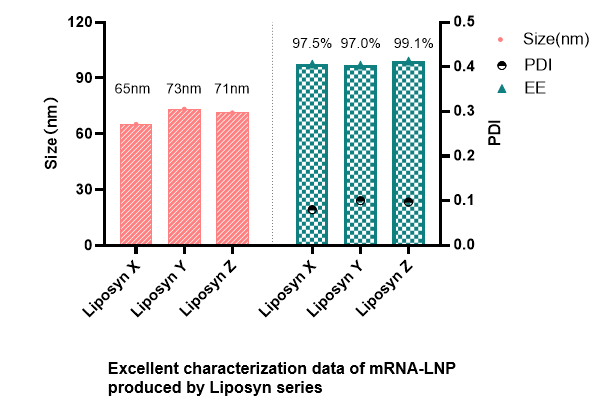
High quality of mRNA-LNP
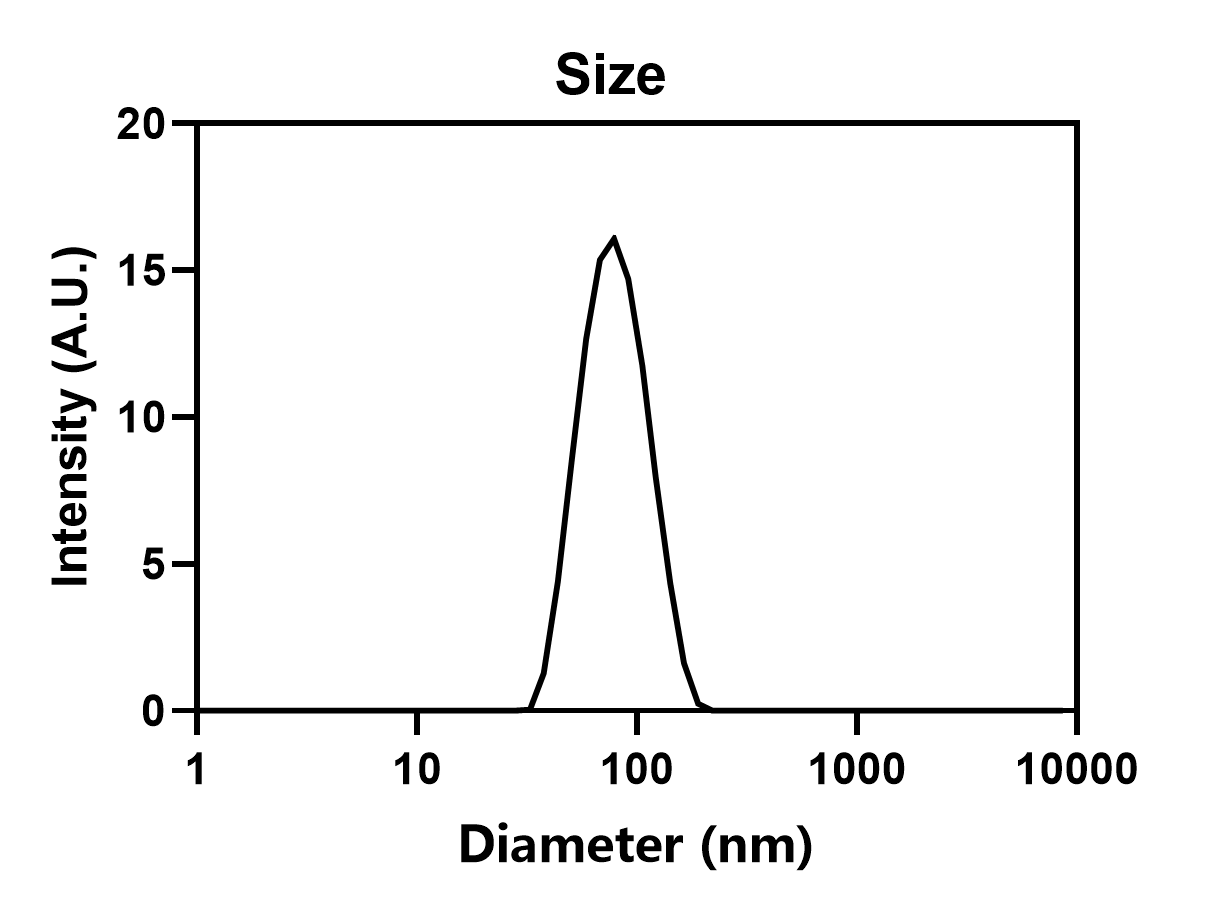
Size: 75.6 nm,polydispersity: 0.10, Encapsulation efficiency: 97%
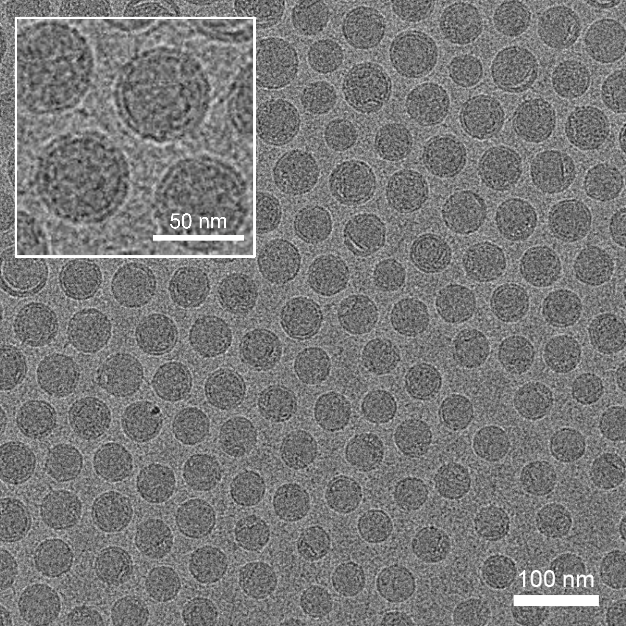
Spherical and uniform shape under cryo-TEM.
Lyophilization
Freeze-drying, or lyophilization refers to the process of sublimating the solvent at low-temperature and vacuum conditions. Lyophilization preserves the spatial structure and biological activity of biomolecules as much as possible. It removes water and oxygen, immobilizes nanoparticles, and reduces their collision in the solution state.
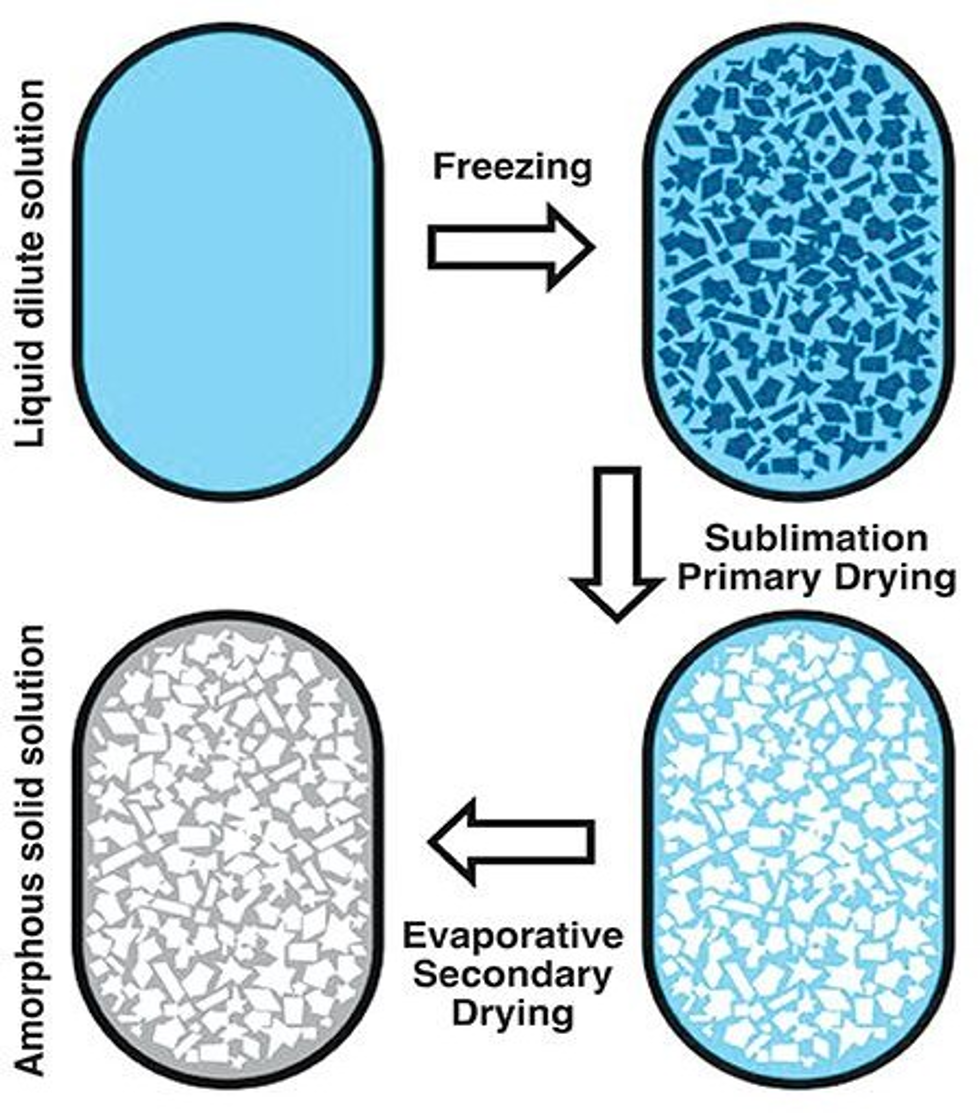
Lyophilization platform of Enobio
Enobio has repeatedly screened and optimized for every detail, and systematically summarized the experience of mRNA-LNP lyophilization, which led to excellent lyophilization result for different mRNA and lipid components.

Lyophilized mRNA-LNP samples
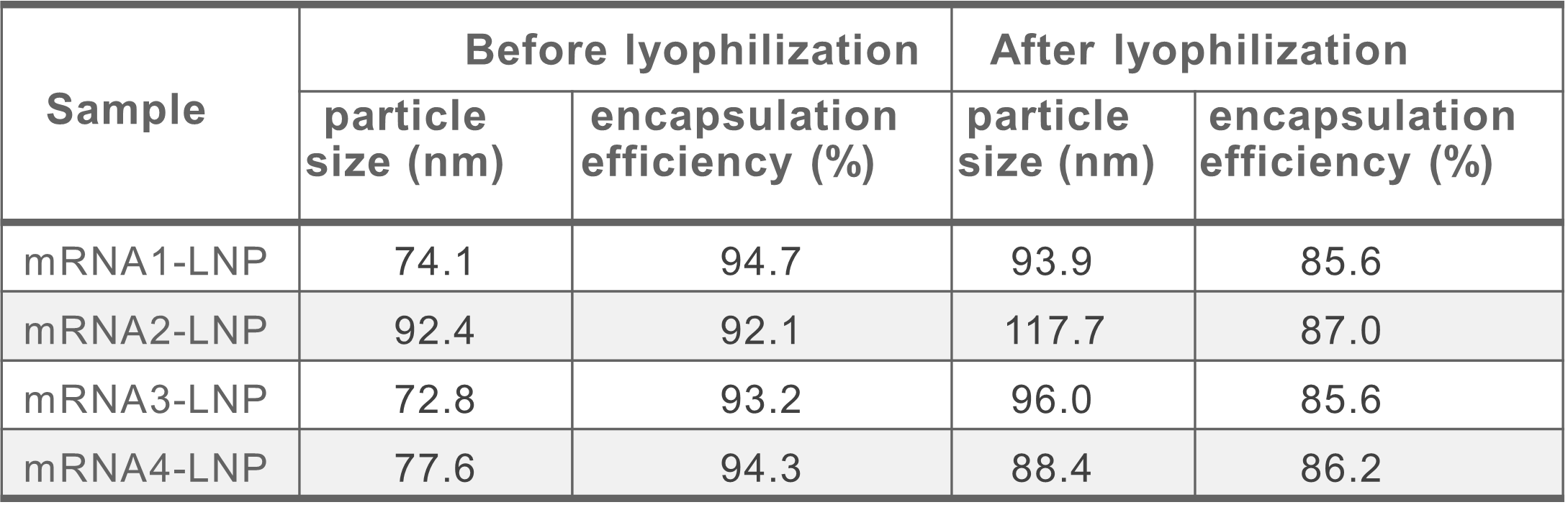
Lyophilization with different mRNAs
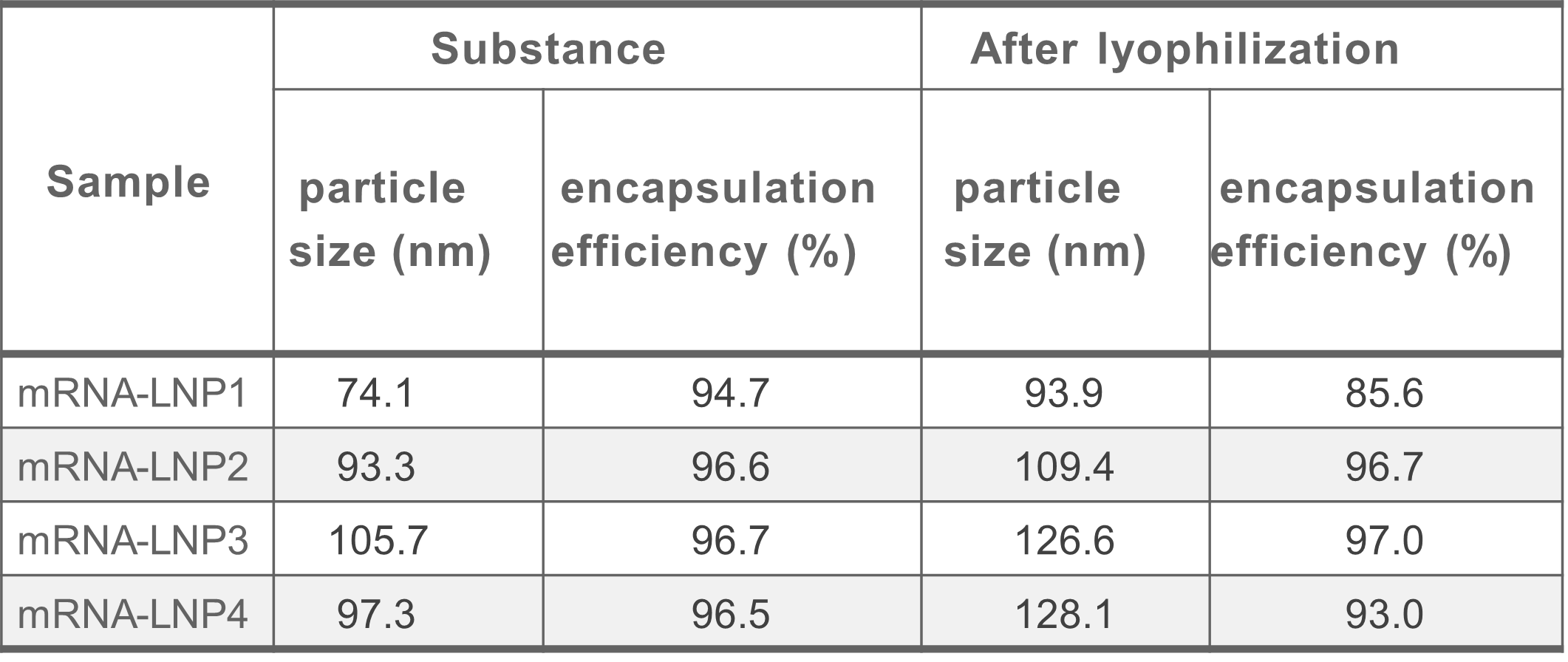
Lyophilization with different LNPs
No significant changes after lyophilization
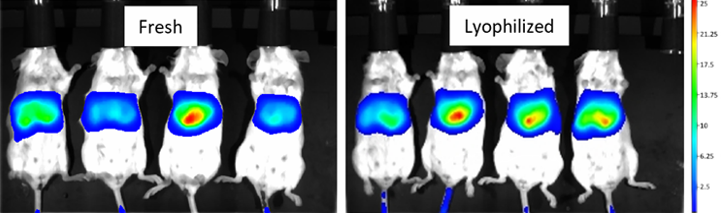
In vivo activity of the lyophilized formulation was not affected
Comparison of Luciferase mRNA-LNP expression efficiency in vivo before and after lyophilization, Balb/c mice were injected with 5 μg of mRNA-Luc LNP in the tail vein, and imaged 24 h later, which showed that there was no significant change in fluorescence brightness before and after lyophilization.
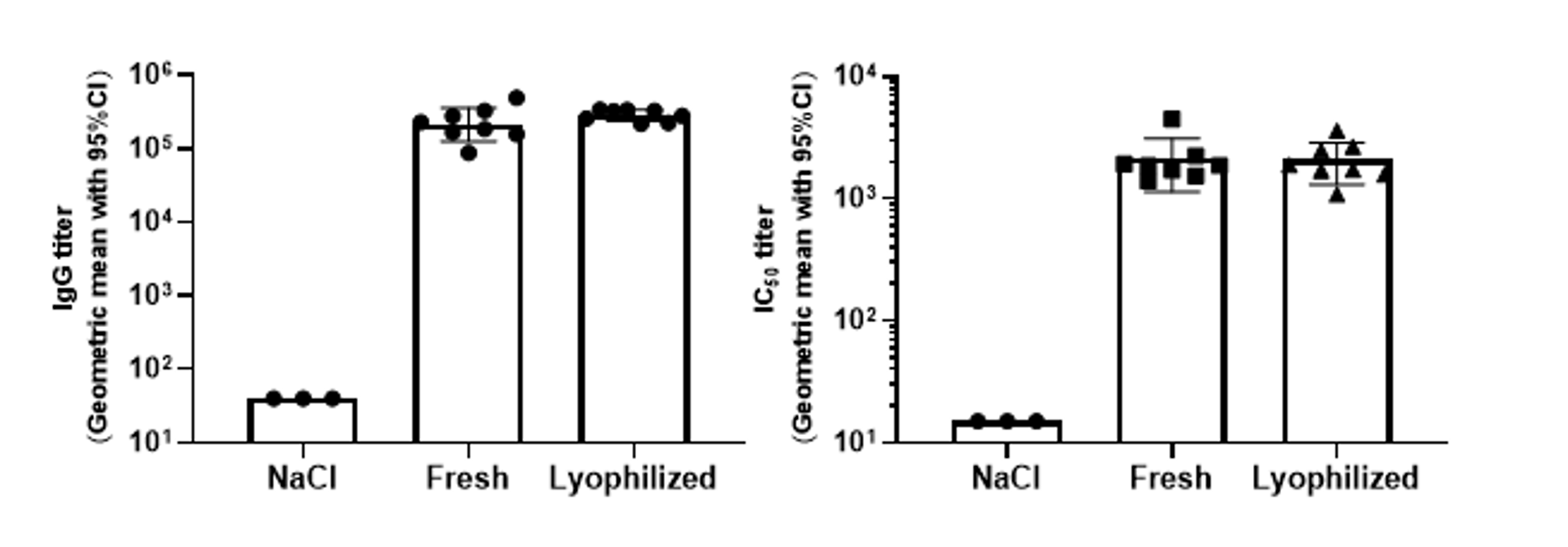
The vaccine still induces the same immune response after lyophilization
Balb/c mice were immunized with 5 μg of mRNA-WT LNP before and after lyophilization of D0/D7, and blood was collected on D21 to detect the titers of binding antibody and pseudovirus-neutralizing antibodies, which showed that there was no difference in immunogenicity before and after lyophilization.
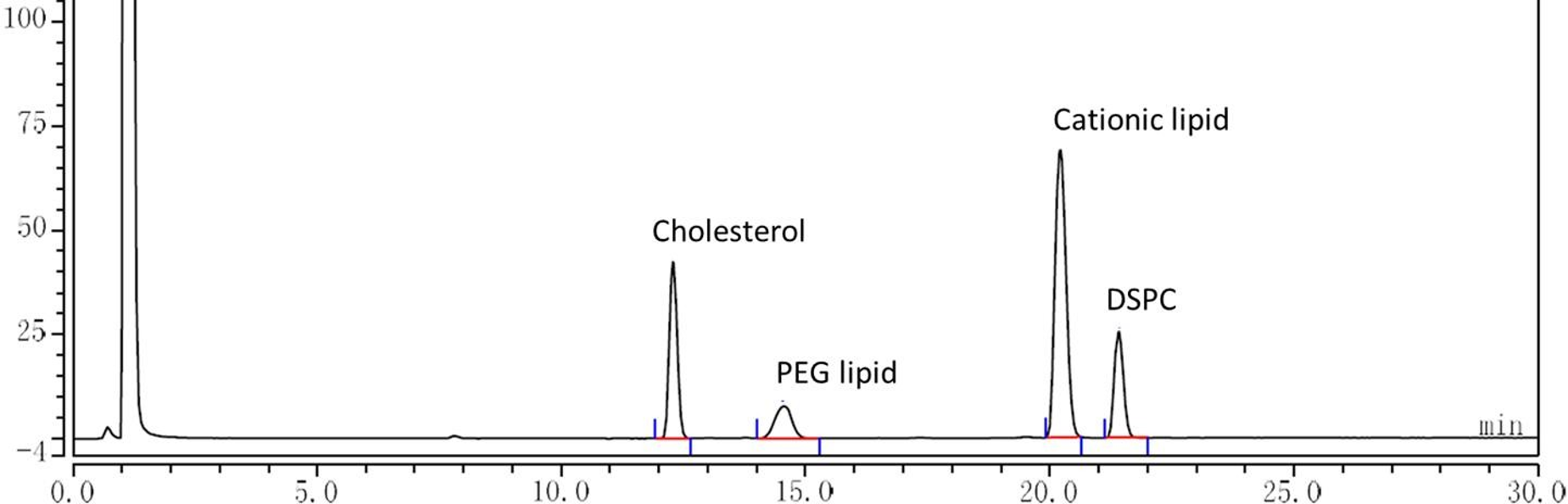
The rate of hydrolytic oxidation of lipids and mRNA decreases dramatically in lyophilized forms
No new impurities were generated by HPLC-CAD analysis of lyophilized mRNA-LNP after 6 months at 25℃.
The mRNA purity of lyophilized mRNA-LNP remained over 93.5% after 6 months at 25 ℃.
Excellent stability of lyophilized samples
Comparison of the stability of lyophilized formulations under different storage conditions.